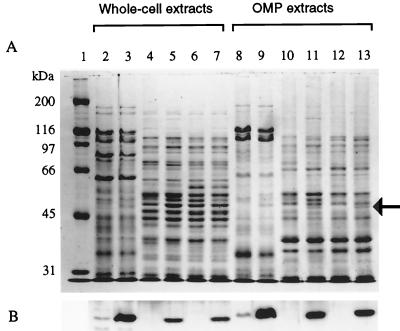
FIG. 2.
Analysis of SmeF expression by bacterial strains containing the smeDEF operon. (A) Protein profiles obtained by SDS-8% polyacrylamide gel electrophoresis of protein extracts from S. maltophilia and E. coli strains either expressing or not expressing or not expressing smeDEF. (B) Results of Western blot analysis of the same samples using an anti-SmeF antibody. In all cases, samples contained 5 μg of protein. Lanes 2 to 7, whole-cell protein extracts; lanes 8 to 13, OMP fractions. Slight differences in protein mobilities were observed between whole-cell extracts and OMP fractions. Lane 1, molecular mass standards; lanes 2 and 8, S. maltophilia D457 strain; lanes 3 and 9, S. maltophilia D457R strain; lanes 4 and 10, E. coli AA81; lanes 5 and 11, E. coli AA81(pAS1); lanes 6 and 12, E. coli AA68; lanes 7 and 13, E. coli AA68(pAS1). The arrow shows the position of SmeF. Note that the protein is expressed only in E. coli strains containing smeDEF genes. Also, SmeF is overexpressed in the MDR S. maltophilia D457R mutant and is detectable, although at a low level, in the wild-type D457 strain.