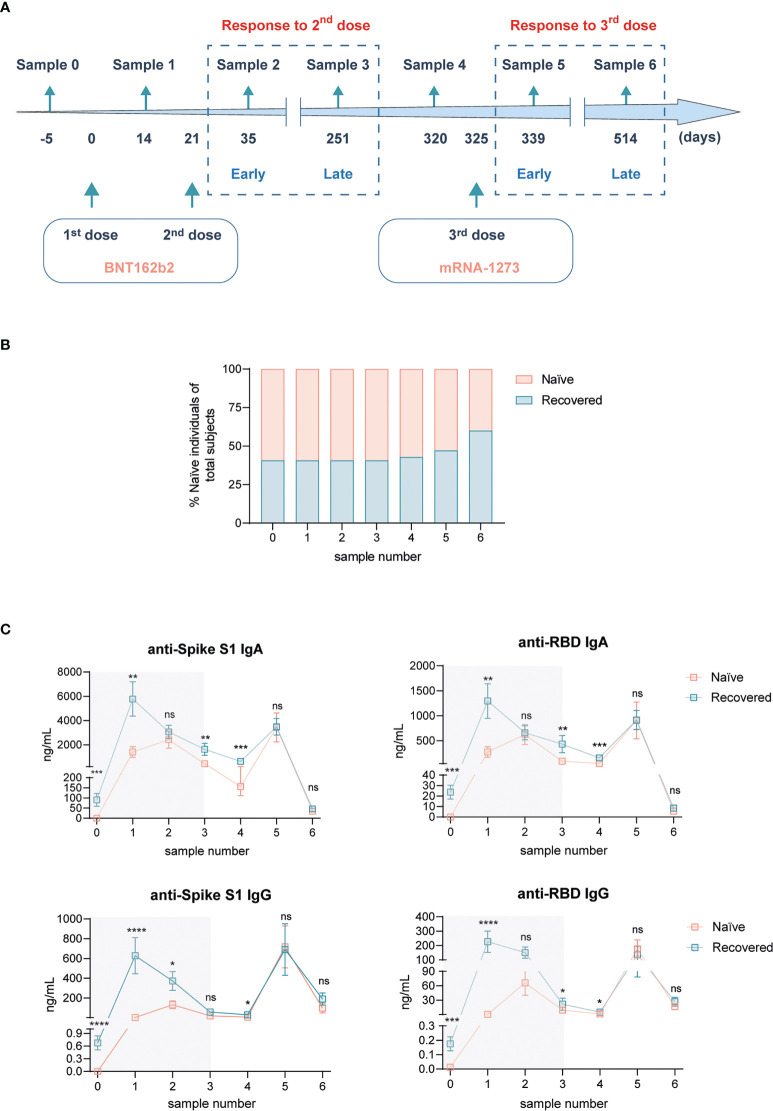
Figure 1.
Humoral response Spike-specific of SARS-CoV-2 subsequent to BNT162b2 mRNA vaccination plus mRNA-1273 booster dose in naïve individuals and individuals recovered from COVID-19. (A) Experimental design. Blood samples from healthy medical personnel were collected 5 days before the first dose of BNT162b2 mRNA vaccine (sample 0), 14 days after the first dose of BNT162b2 mRNA vaccine (sample 1), 14 days (sample 2, 35 days from the beginning), 230 days (sample 3, 251 days from the beginning), and 69 days (sample 4, 320 days from the beginning) after the second dose of BNT162b2 mRNA vaccine. Subsequent blood samples were collected 14 days (sample 5, 339 days from the beginning) and 189 days (sample 6, 514 days from the beginning) following the administration of the mRNA-1273 booster dose. (B) Frequency of naïve participants and recovered from COVID-19 along the study. (C) Plasmatic levels of anti-Spike S1 IgA (upper left panel), anti-receptor binding domain (RBD) IgA (upper right panel), anti-Spike S1 IgG (lower left panel), and anti-RBD IgG (lower right panel) antibodies. Shadowed areas represent analyses performed in (5). n = 16 naïve, n = 11 recovered from COVID-19 at sample 0. Data in (C) are shown as mean ± SEM. Unpaired Student’s t-test in each sample number between naïve and COVID-19-recovered subjects (ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001). In every plot, all the possible experimental conditions were compared; statistically significant differences are indicated, and the lack of statistical indication means the absence of a significant difference.