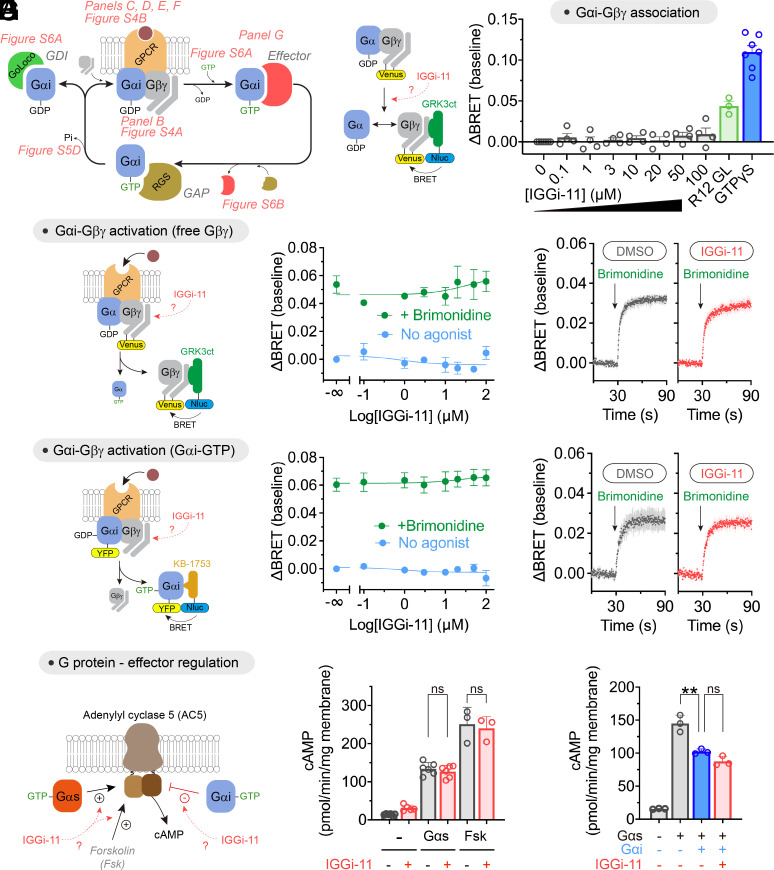
Fig. 3.
Lack of effect of IGGi-11 on G-protein coupling to GPCRs and effectors. (A) Diagram of key steps and protein interactions involved in Gαi-subunit functions. (B) IGGi-11 does not dissociate Gβγ from Gαi3 in membranes isolated from HEK293T cells expressing a BRET-based biosensor for free Gβγ, whereas two positive controls do (a GoLoco peptide derived from RGS12, R12 GL, 25 μM; and GTPγS 300 μM). (C–F) IGGi-11 does not affect GPCR-mediated activation of Gi3 as determined by the dissociation of Gαi3–Gβγ heterotrimers (C and D) or the formation of Gαi3–GTP (E and F) using BRET-based biosensors. In C and E, membranes isolated from HEK293T cells expressing the α2A adrenergic receptor were treated with the indicated concentrations of IGGi-11 with (green) or without (blue) stimulation with a receptor agonist (brimonidine, 1 μM) for 2 min before BRET measurements. In D and F, BRET was continuously measured in real time in the presence of 100 μM IGGi-11 or vehicle (1% dimethyl sulfoxide (DMSO), v:v). (G) IGGi-11 does not interfere with G-protein-mediated regulation of adenylyl cyclase. Membranes isolated from HEK293T cells expressing adenylyl cyclase 5 were treated with IGGi-11 (100 μM), purified Gαs (0.5 μM), purified myristoylated Gαi1 (Gαi, 1 μM), and forskolin (Fsk, 10 μM) in the combinations indicated in the graphs. Mean ± SEM (N ≥ 3). **P < 0.01, ANOVA.