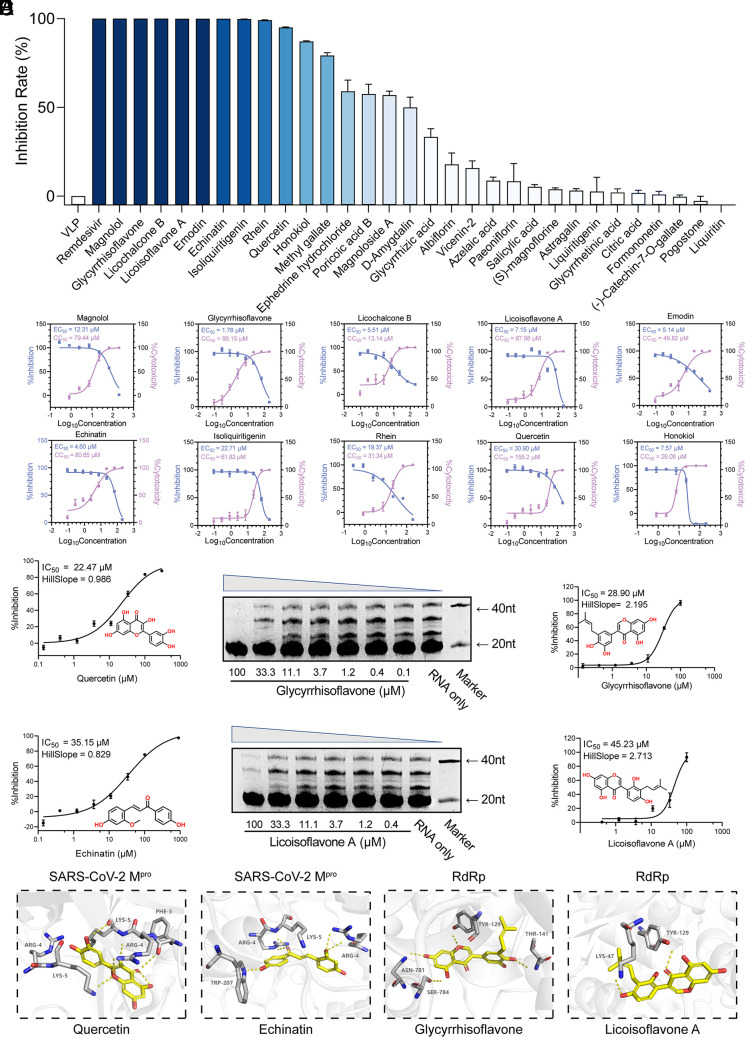
Fig. 3.
Screening of antiviral compounds from Q-14. (A) Preliminary screening of commercially available prototype compounds with antiviral activity at 100 μM based on a packaging cell line for ectopic expression of the nucleocapsid (Caco-2-N) infected with SARS-CoV-2-like-particles (trVLPs). (B) The 50% effective concentration (EC50) and 50% cytotoxic concentration (CC50) values of the top 10 compounds. The antiviral activity (blue) and cytotoxicity (violet) were measured. (C) SARS-CoV-2 Mpro inhibition activities of quercetin and echinatin by a fluorescence resonance energy transfer (FRET)–based protease assay. (D) The inhibition of RdRp by glycyrrhisoflavone and licoisoflavone A measured using an in vitro polymerase activity assay. (E) Molecular docking of quercetin and echinatin on SARS-CoV-2 Mpro and glycyrrhisoflavone and licoisoflavone A on RdRp. SARS-CoV-2 Mpro and RdRp are shown in gray cartoon representation, essential residues in gray sticks, and four compounds (quercetin, echinatin, glycyrrhisoflavone, and licoisoflavone A) in yellow sticks. The polar interactions between the compounds and SARS-CoV-2 Mpro/RdRp are shown in yellow dashes. Data are expressed as the mean ± SEM. Experiments were repeated in triplicate, independently.