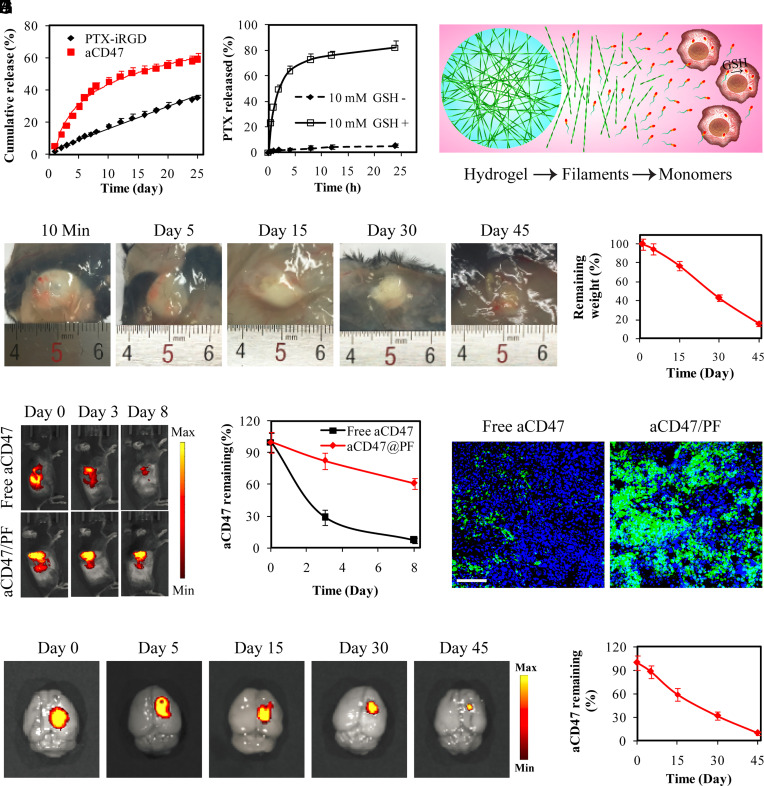
Fig. 2.
Supramolecular PTX PF hydrogel scaffold extends local retention and release of aCD47 in situ. (A) Cumulative release profiles of PTX–iRGD and aCD47 from aCD47/PF supramolecular hydrogels. aCD47 was labeled with FITC. (B) Free PTX release profiles of 250 μM PTX–iRGD solution incubated with or without 10 mM GSH. (C) Schematic illustration of the proposed PTX release mechanism: hydrogel disruption, PTX PF dissociation followed by liberating the parent PTX in intracellular GSH reductive environment. (D) Images showing in vivo gelation and degradation of PTX–iRGD supramolecular hydrogels in C57BL/6 mice. (E) Quantification of the degradation profile of the PTX–iRGD supramolecular hydrogel. Data are given as mean ± SD (n = 3). (F) Fluorescent IVIS images showing the retention of aCD47 in the tumor at the indicated time points following intratumoral injection with free aCD47 or aCD47/PF solution, aCD47 was labeled with Cy5.5. (G) Quantification of the tumoral retention profile of aCD47. Data are given as mean ± SD (n = 3). (H) Fluorescence images of tumor sections from GL-261 tumor-bearing mice that were locally treated with free aCD47 or aCD47/PF on day 3. Green: FITC-labeled aCD47, Blue: DAPI-stained nuclei. (Scale bar: 200 μm.) (I) Ex vivo fluorescence images of brains intracranially injected with aCD47/PF confirmed the retention of aCD47 in the brain. aCD47 was labeled with Cy5.5. (J) Quantification of the retention profile of aCD47 in the brain. Data are given as mean ± SD (n = 3).