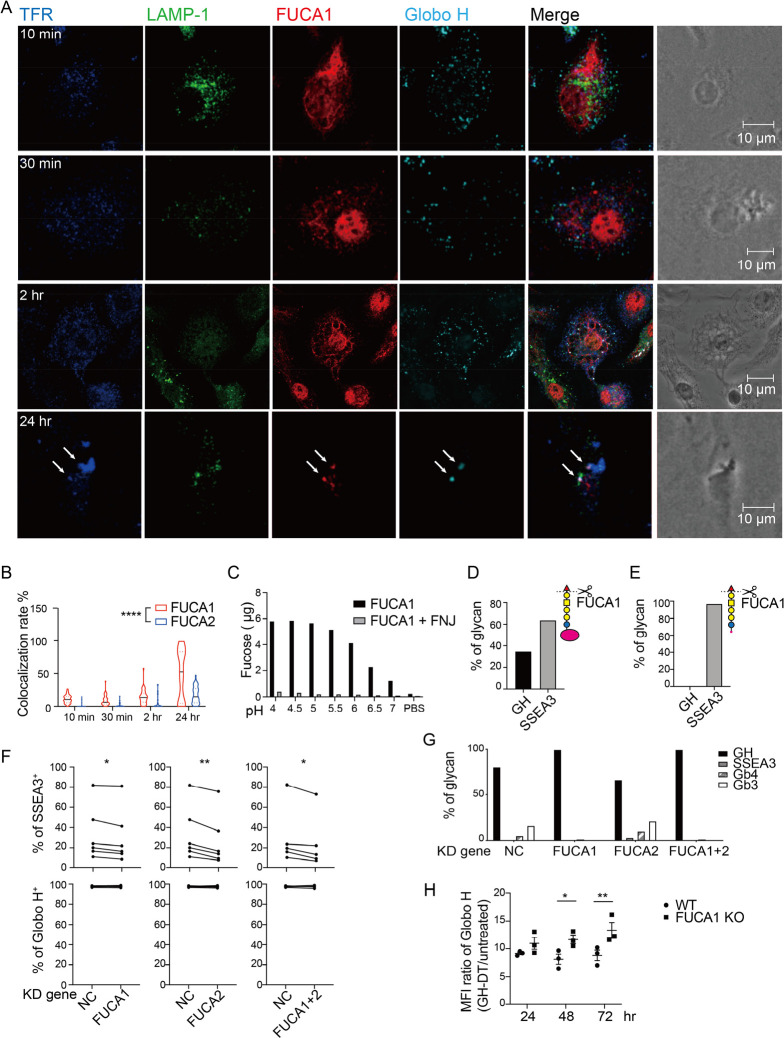
Figure 5.
GH-DT is processed by FUCA1 to SSEA3 glycan in BMDCs. (a) GH glycan was colocalized with FUCA1 in BMDCs within 24 h after treatment with GH-DT. TFR and LAMP-1 represent transferrin receptor and lysosomal-associated membrane protein 1, respectively. Arrows indicate early endosomes containing both FUCA1 and GH glycan. (b) The colocalization rate between GH glycan and FUCA1 was higher than that between GH glycan and FUCA2. 45 cells per group were counted. (c) Recombinant FUCA1 digested GH glycan with the highest activity at pH = 4.5. Addition of FUCA1 inhibitor, fuconojirimycin (FNJ), inhibited the digestion. (d) Oxidative release of glycan and LC–MS/MS analysis showed that FUCA1 partially hydrolyzed GH glycan on GH-DT and (e) FUCA1 completely hydrolyzed GH glycan on GH-peptides in vitro. (f) The presentation of SSEA3 glycan was reduced in FUCA1 or/and FUCA2 knockdown BMDCs compared to the negative control (NC) BMDCs. Data were analyzed by paired t tests. (g) Oxidative release of glycans and LC–MS/MS analysis showed that the level of digested glycans was reduced in FUCA1 knockdown BMDCs. (h) BMDCs with knockdown of FUCA1 presented more GH glycans on the cell surface as compared to WT BMDCs. Results in (h) are mean ± SEM (n = 3). * p < 0.05, ** p < 0.01, **** p < 0.0001.