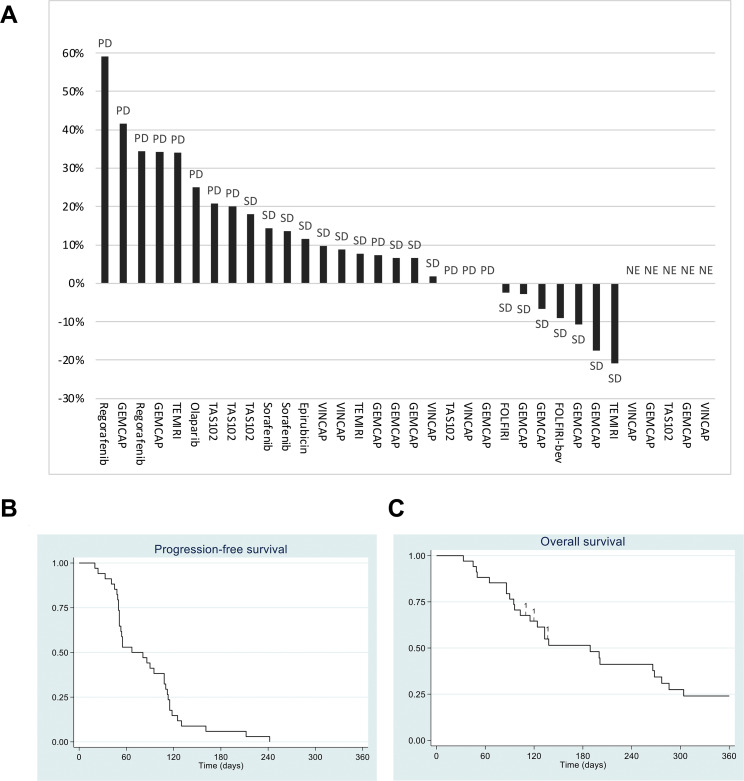
Fig. 2.
(A) The best response to systemic treatment during the treatment period in 29 patients with measurable disease and five patients with non-evaluable disease according to the Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST 1.1): percentage change in tumor burden. PD=progressive disease, SD=stable disease, GEM=gemcitabine, CAP=capecitabine, TEM=temozolamide, IRI=irinotecan, VIN=vinorelbine, FOLF=5-flourouracil and leucovorin, bev=bevacizumab. NE=non evaluable disease: one had no measurable disease at baseline, and four had clinical progression or death before the first scanning evaluation). Kaplan-Meier curves showing progression-free survival (B) and overall survival (C) of all 34 patients in the precision cohort. No patients were censored for PFS, and five patients still alive at the date of analysis were censored (two after 360 days)