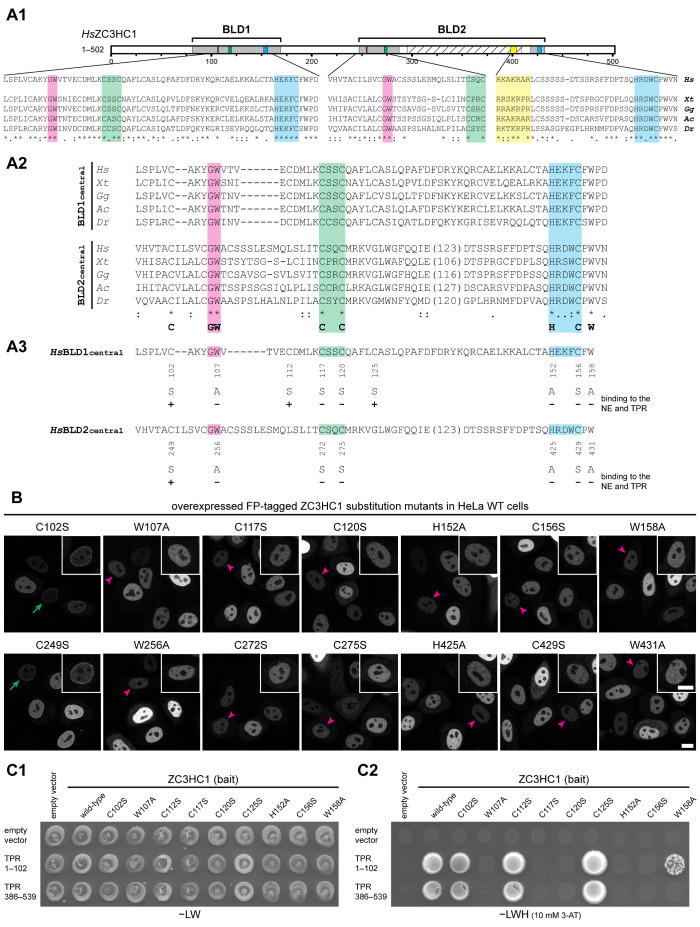
FIGURE 2:
Specific amino acids within both BLDs of ZC3HC1 are essential for the initial binding to TPR. (A) Schematic depiction of the two BLDs, sequence alignments of representative vertebrate homologues, and an overview of the single-aa-substitution mutants of FP-tagged HsZC3HC1. (A1) Schemes of the two HsZC3HC1 BLDs and alignment of the vertebrate homologue sequence segments corresponding to the minimal central region of each BLD, including the G-W, C-X(2)-C, and H-X(3)-C peptides, and some flanking residues. Sequences are from the human homologue (Hs), amphibians (Xenopus tropicalis, Xt), birds (Gallus gallus, Gg), reptiles (Anolis carolinensis, Ac), and fish (Danio rerio, Dr). Areas highlighted in addition to those in Figure 1C represent G-W dipeptides (magenta) and the NLS (yellow). (A2) Alignments between sequences representing the central BLD1 region and corresponding BLD2 segments but excluding the BLD2-specific sequence insertions (variable lengths in brackets). The bottom line provides a minimal sequence signature identical for both BLDs in these vertebrate homologues. The HsZC3HC1 BLD1 and BLD2 sequences shown represent L97–D160 and V244–N433, respectively. The inner boundaries flanking the BLD2 insertion correspond to E288 and D412. (A3) Individual aa substitutions within the BLD regions and their effects on NE binding and TPR interaction. (B) Fluorescence microscopy of WT HeLa cells transiently transfected with a selection of expression vectors encoding full-length HsZC3HC1 mutants, each carboxy-terminally tagged with EGFP and carrying one of the single-aa substitutions specified in A3. Representative cells with low expression levels are marked as in Figure 1B2 by green arrows and magenta arrowheads, with insets showing the magnified and signal-enhanced images of the marked cells. Bar, 10 µm. (C) Y2H experiments analyzing the interaction of the single-aa-substitution mutants of HsZC3HC1 with two HsTPR segments that include ZC3HC1 interaction domains. (C1) Representative colony growth of diploid cells expressing TPR segments together with WT ZC3HC1 or some of its mutants. Cells were grown on a selection medium lacking leucine and tryptophan (−LW). (C2) Visualization of Y2H interactions after replica-plating onto –LW selection medium also lacking histidine (−LWH) and supplemented with 3-AT. Note that those single-aa-substitution mutants of ZC3HC1 that did not impair NE association in HeLa cells (e.g., C102S, C112S, and C125S) allowed colony growth when paired with the ZC3HC1-binding domains of TPR. By contrast, no colony growth was observed for the mutants incapable of associating with the NE (e.g., C117S, C120S, and C156S). Further note that mutant W158A, which associated with the NE in ZC3HC1 KO cells (Supplemental Figure S2B2) but not in WT cells (as shown in B), was capable of an attenuated TPR interaction (see also Supplemental Figure S2E).