Sir,
Persistent granulomatous hypersensitivity reaction is a rare but familiar adverse effect of Mycobacterium indicus pranii (MIP)/Mycobacterium welchii (Mw) immunotherapy. We describe a prolonged course of this adverse effect and ways to avoid and treat it.
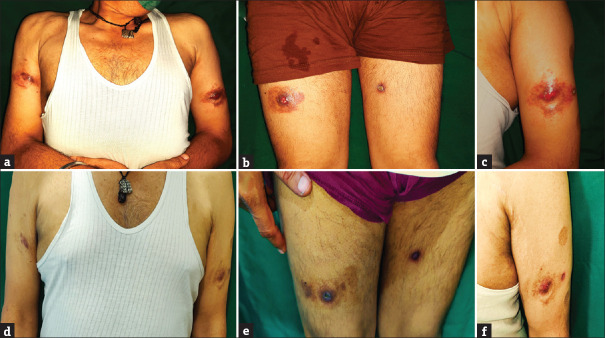
A 56-year-old male presented with multiple painful erythematous pus-discharging ulcerated nodular swellings at bilateral arms and thighs for 4 months [Figure 1a–c]. On palpation, the nodules were tender, with variable consistency ranging from soft to firm in different areas. The nodules appeared when the patient received MIP injections (Sepsivac, Cadila Pharmaceuticals Limited, Ahmedabad, India) for severe COVID-19 infection while he was admitted to an intensive care unit. The nodules appeared 2 days after receiving the first injection at three sites over the bilateral thighs. They also appeared simultaneously at three other sites over bilateral arms where the patient was injected with the same medication 3 days before the thigh injections. In total, the patient developed the nodules at six injected sites, 2 days after the second dose of injections. With a clinical diagnosis of exaggerated hypersensitivity reaction to MIP immunotherapy, a biopsy was done which showed a mixed cell granulomatous tissue reaction pattern [Figure 2]. No acid-fast bacilli were seen in special staining and tissue culture.
Figure 1.
Exaggerated hypersensitivity reaction to Mycobacterium indicus pranii at the injection sites. (a) Painful tender erythematous pus-discharging ulcerated nodules at the arms, and (b) thighs. (c) Close-up view. (d–f) Resolution after 4 months of treatment with minocycline and topical steroids
Figure 2.
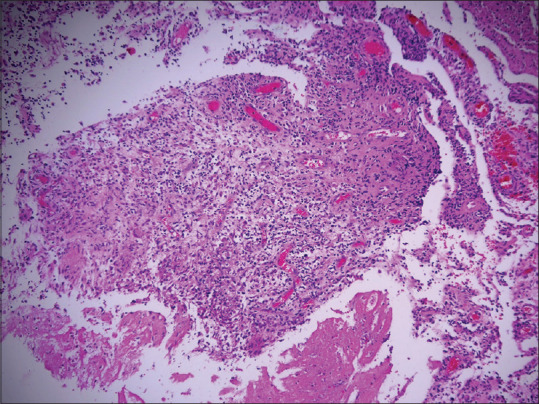
Mixed cell fibrosing granulomatous tissue reaction with lymphocytes, histiocytes, some plasma cells, few neutrophils, eosinophils and Langhans giant cells with some fibrosis (H&E,100×)
Because of persisting pus discharge, pain and tenderness, the patient was started on minocycline 100 mg once daily and mid-potent topical steroids, with which the lesions gradually resolved after 4 months [Figure 1d–f], but recurred after a further 4 months. Recurred lesions also resolved after 6 weeks of repeat minocycline, and the patient is free of lesions for further 5 months of the follow-up period.
Various treatment modalities have been used for the management of SARS-CoV-2 and its complications with uncertain benefits. MIP, also known as Mw, is a non-pathogenic member of the Mycobacterium avium complex that has good immunoprophylactic and immunotherapeutic properties against pathogenic mycobacteria including those causing tuberculosis and leprosy. It has also been used in severe sepsis, including COVID-19 sepsis, as an immunomodulator by potentially decreasing the overexpressed pro-inflammatory cytokines, increasing the Th1 type of cross-reactive response against SARS-CoV-2 and inducing an adaptive immune response for clearing the virus.[1] The proposed regimen is 0.3 ml/day of the MIP vaccine, in three divided doses of 0.1 ml each, at three different sites, given intradermally for three consecutive days.[1] Subcutaneous route is not recommended.
Almost all patients (31, 97%) out of 32 who received the MIP vaccine for cutaneous warts in a study developed nodules at sites of injection, 16 (50%) of them also had ulceration and 5 (15.6%) had pus discharge.[2] In another series of eight patients who developed nodules with or without ulceration and pus discharge after MIP use, lesions were confined to the sites of current or previous injections, which ranged from 3 to 15 (mean 8) per patient.[3] Onset of these lesions was mostly after fourth to fifth cycle of immunotherapy. Histopathology showed a mixed cell granulomatous reaction pattern similar to our patient and broken granular acid-fast bacilli were identified only in two cases. Mycobacterial culture was sterile in all patients. Similar cases have been reported when MIP has been used for COVID-19 sepsis.[4,5] Development of ulcerating nodules in such cases has been ascribed to injection being administered subcutaneously instead of intradermally.[5]
A variety of treatments have been tried for these nodules, including multidrug antibiotic regimens used for treating non-tubercular mycobacterial infections.[2] Minocycline is a safer monotherapy for these persistent granulomatous hypersensitivity reactions and leads to resolution within 6–12 weeks with post-inflammatory hyperpigmentation or erythema.[3]
This case is unique for having an early onset after 5 days with just two cycles of injections, a very severe and prolonged course, requirement of treatment for many months for resolution, recurrence at the same sites after resolution and resolution of recurrence with the same therapy. The route of injection in this patient was unknown. Accidental subcutaneous injection could have been responsible for early, severe and prolonged course. As dermatologists are most familiar with MIP and its adverse effects, they can guide their colleagues from other disciplines who are using it for COVID, on how to avoid and manage such hypersensitivity reactions.
Patient consent
Written informed consent was obtained from the patient.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sehgal IS, Guleria R, Singh S, Siddiqui MS, Agarwal R. A randomised trial of Mycobacterium w in critically ill patients with COVID-19: ARMY-1? ERJ Open Res. 2021;7:00059–02021. doi: 10.1183/23120541.00059-2021. doi: 10.1183/23120541.00059-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandra S, Sil A, Datta A, Pal S, Das NK. A double-blind, randomized controlled trial to compare the effectiveness and safety of purified protein derivative of tuberculin antigen with Mycobacterium w vaccine in the treatment of multiple viral warts. Indian J Dermatol Venereol Leprol. 2019;85:355–66. doi: 10.4103/ijdvl.IJDVL_549_18. [DOI] [PubMed] [Google Scholar]
- 3.Vinay K, Narang T, Saikia UN, Kumaran MS, Dogra S. Minocycline successfully treats exaggerated granulomatous hypersensitivity reaction to Mw immunotherapy? Dermatol Ther. 2017;30 doi: 10.1111/dth.12452. doi: 10.1111/dth.12452. [DOI] [PubMed] [Google Scholar]
- 4.Jakhar D, Sah N, Kaul S. Injection site reactions associated with the use of Mycobacterium w in COVID-19 patients. Int J Dermatol. 2022;61:642–3. doi: 10.1111/ijd.16104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawla RK, Chawla AK, Chaudhary G, Chopra K, Chawla MK. Mycobacterium W.-An unusual side effect. Indian J Tuberc. 2022;69:250–2. doi: 10.1016/j.ijtb.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]