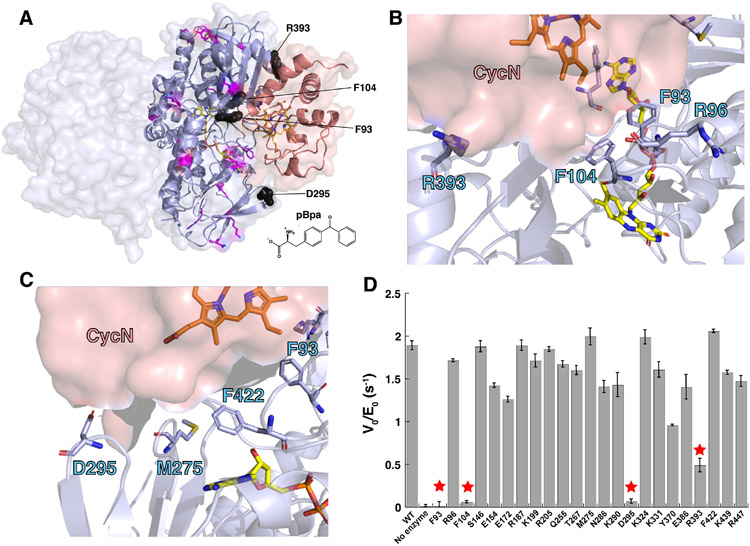
Figure 2.
(A) Structure of the NicA2-CycN complex predicted by AlphaFold2 Multimer. The NicA2 homodimer is shown in light blue and CycN is shown in salmon. Residue positions replaced with pBpa that had minimal impact on the rate of electron transfer from nicotine to CycN are shown as sticks in magenta. Residue positions replaced with pBpa that substantial decreased the rate of electron transfer from nicotine to CycN are shown as black spheres. The chemical structure of pBpa is shown for reference. (B) and (C) Orientation of side chains for select residues in NicA2 at the predicted CycN binding site that were mutated to pBpa in this study. (D) Measured rate of electron transfer from nicotine to CycN by various pBpa-containing mutants of NicA2. Residue positions that dramatically decreased the rate of electron transfer upon replacement with pBpa are labeled with stars.