Abstract
Background
Real-world data on early treatment of coronavirus disease 2019 (COVID-19) outpatients with newly approved therapies are sparse.
Aim
To explore the pattern of use of monoclonal antibodies (mAbs)/antiviral therapies approved for early COVID-19 treatment in non-hospitalized patients from England and Italy from December 2021 to October 2022.
Methods
Public national dashboards on weekly mAb/antiviral use and/or severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection diagnoses from the Italian Medicines Agency, the Italian National Institute of Health, National Health Service in England and the UK Government were explored. Prevalence of antiviral use in outpatients during the entire study period and every two weeks was calculated, as a whole and by class and compounds. An interrupted time-series (ITS) analysis was carried out to assess the impact of predominant SARS-CoV-2 variants over time on the prevalence of use of mAbs/antivirals in England and Italy.
Results
Overall, 77,469 and 195,604 doses of mAbs/antivirals were respectively administered to a total of 10,630,903 (7.3 per 1000) and 18,168,365 (10.8 per 1000) patients diagnosed with SARS-CoV-2 infection in England and Italy. Prevalence of use every two weeks increased from 0.07% to 3.1% in England and 0.9% to 2.3% in Italy during the study period. Regarding individual compounds, sotrovimab (prevalence of use, 1.6%) and nirmatrelvir/ritonavir (1.6%) in England, and nirmatrelvir/ritonavir (1.7%) and molnupiravir (0.5%) in Italy, reported the highest prevalence during a 2-week period. In the ITS analysis, the transition from Delta to Omicron variant predominance was associated with a significant increase in the use of sotrovimab, molnupiravir, remdesivir and nirmatrelvir/ritonavir in both England and Italy, with a reduction of other marketed mAbs. The extent of the increase was higher in England than in Italy for all these drugs except for nirmatrelvir/ritonavir.
Conclusions
In this dual nationwide study, the prevalence of use of mAbs/antivirals against SARS-CoV-2 for early outpatients’ treatment increased slowly up to 2.0–3.0% of all patients diagnosed with SARS-CoV-2 infection in both England and Italy from December 2021 to October 2022. The trend of individual drug use varied in relation to predominant SARS-CoV-2 variants with some differences across countries. In line with scientific societies’ guidelines, nirmatrelvir/ritonavir was the most frequently prescribed antiviral in both countries in the most recent period.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40259-023-00601-w.
Key Points
| The trend of monoclonal antibody (mAb)/antiviral use varied in relation to predominant SARS-CoV-2 variants. |
| The prevalence of use of mAbs/antiviral therapies for early treatment of COVID-19 outpatients increased slowly in England and in Italy. |
| Nirmatrelvir/ritonavir was the most frequently prescribed/administered antiviral in the most recent period. |
Introduction
Coronavirus disease 2019 (COVID-19) is a respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first detected in patients with pneumonia in December 2019 [1]. Overall, more than 6.8 million people worldwide died due to the pandemic [2] at the time of writing. At the beginning of the pandemic, repurposing of several drugs as potential antivirals against SARS-CoV-2 was proposed [3]. Since May 2020 several newly developed antivirals for early treatment of COVID-19 were introduced into the market based on the accelerated regulatory pathway, due to successfully completed premarketing clinical studies [4–7]. Among these, a few monoclonal antibodies (mAbs) against the spike protein of SARS-CoV-2 (regdanvimab, sotrovimab, casirivimab/imdevimab, tixagevimab/cilgavimab and bamlanivimab, either as monotherapy or in combination with etesevimab) have been authorized (Supplementary Table 1), with either full or conditional approval specifically for early treatment of COVID-19 outpatients not requiring supplemental oxygen therapy and at high risk of progressing to severe disease. In addition, three antiviral drugs with different mechanisms of action have been authorized under conditional approval for the same indication of use: remdesivir (which was already authorized for treatment of hospitalized patients with COVID-19), nirmatrelvir/ritonavir and molnupiravir. Of note, recently the European Agency of Medicines (EMA) recommended the refusal of the marketing authorization for molnupiravir [8]. The emergence of new SARS-CoV-2 variants has reduced the benefits of mAbs over time, leading to country-specific health policy interventions that restricted the use of some of these drugs for the early management of COVID-19 outpatients [9].
So far, no nationwide studies comparing the trend in the use of mAbs and antivirals against SARS-CoV-2 in an outpatient setting during different COVID-19 pandemic waves in England and Italy have been published. This drug utilization study aimed to explore and compare the pattern of mAb and antiviral drug use for the early treatment of SARS-CoV-2 infection-diagnosed outpatients in England and Italy from December 2021 to October 2022.
Methods
Data Source
A descriptive, retrospective study was conducted using publicly available SARS-CoV-2 infection pandemic monitoring dashboards/reports from England and Italy, which provide nationwide updated data on SARS-CoV-2 infection cases and related deaths, and mAbs/antivirals drug use over time in each country. Drug utilization data were specifically retrieved from public reports published on the web pages of the National Competent Authorities of each country, which included information provided by National Healthcare Service hospitals and COVID-19 medicine delivery units treating patients when an individual is judged clinically eligible for treatment. For Italy weekly mAb/antiviral monitoring reports were available on the Italian Medicines Agency website from April 2021 to October 2022 [5, 6], while for England on the National Health Service website from December 2021 to October 2022 [10]. The UK Government COVID-19 Dashboard [11] and the Italian National Institute of Health COVID-19 Dashboard [12] were additionally searched to identify the number of SARS-CoV-2 infection confirmed diagnoses in England and Italy, respectively, during the same observation period. Confirmed cases are collected consistently across countries on the basis of the European Centre for Disease Prevention and Control official case definition for COVID-19 [13]: SARS-CoV-2-positive nucleic acid amplification test or antigen-based rapid diagnostic tests using nasopharyngeal swabs. Subjects with repeated swabs to assess the clinical course are counted only once. Information on the distribution of SARS-CoV-2 variants across European countries over time was obtained from surveillance bulletin provided by the European Centre for Disease Prevention and Control and World Health Organization (WHO) Regional Office for Europe [14].
Study Drugs
The following therapies for the early treatment of COVID-19 outpatients were included in the study: (a) mAbs: bamlanivimab, bamlanivimab/etesevimab, casirivimab/imdevimab, sotrovimab, regdanvimab and tixagevimab/cilgavimab; and (b) antivirals: remdesivir, molnupiravir and nirmatrelvir/ritonavir. As regards remdesivir, which has been initially authorized for treatment of hospitalized patients with COVID-19, only the use for the early treatment of COVID-19 outpatients was considered. Regarding tixagevimab/cilgavimab, which is also approved as prophylactic treatment of COVID-19 in immunocompromised patients, only the use for early COVID-19 treatment was considered.
Data Analysis
First, the number of administrations (i.e. treatment cycle) of the studied mAbs and antivirals for early treatment of COVID-19 outpatients in England and Italy, in relation to the number of SARS-CoV-2 infection diagnoses during the country-specific study period, was counted. Second, the prevalence of use (%) every two weeks of mAbs and antivirals as a whole and by individual compound in patients diagnosed with SARS-CoV-2 infection was calculated for England and Italy, separately. This was carried out using the number of administered treatments of any or each individual antiviral in outpatients during two consecutive weeks as the numerator, and the number of SARS-CoV-2-positive tests (including both symptomatic and asymptomatic subjects) during the same observation period as the denominator. The prevalence of use of mAbs and antivirals every two weeks was shown graphically in relation to the distribution of SARS-CoV-2 variants. To evaluate the impact of health policy interventions on the rate of an outcome in a defined population using observational data, quasi-experimental designs are the best approaches [15]. For this reason, an interrupted time-series (ITS) analysis with a quasi-Poisson generalized additive model [16–19] was finally performed to assess any statistically significant changes in the level of prevalence of use of each study drug in England and Italy after the final period of Delta SARS-CoV-2 variant predominance and the transition to the Omicron variant period. In the modelling phase we included the count every 2 weeks of the mAb/antiviral drug users as the outcome and the SARS-CoV-2 infection diagnoses every 2 weeks as offset to convert the count outcome in prevalence. We also included a nonlinear function of time (every two weeks) in the model to adjust for temporal trends and seasonality. As output of the modelling results, we estimated the level change parameter and its exponential transformation (prevalence ratio) representing the immediate and lasting effect of the new period (i.e. the end of the Delta variant) on mAb/antiviral use. Negative values (prevalence ratio < 1) suggested a reduction in the use of the study drugs with the end of Delta variant predominance compared with the pre-end of Delta segment, while positive values (prevalence ratio > 1), suggested an increase. We then compared the prevalence ratio of each individual mAb and antiviral in England versus Italy. As for Italy the information on mAbs and antivirals use during the period April 2021 to December 2021 was also available, we estimated only in this country the impact of Omicron SARS-CoV-2 variant emergence on the overall prevalence of use of mAbs and antivirals considering two segments: pre-Omicron variant period (April 2021 to December 2021) and the Omicron variant predominance (December 2021 to October 2022). In addition to the level change parameter, we estimated for each period the slope change parameter and the prevalence ratio, representing the step change over the period of interest [16].
The scatter plot of the prevalence of use and the trend estimated by the model was represented for each analysis. Significance of the parameters was defined as a p-value < 0.05. The models were fitted separately for each drug or combination of drugs. Data processing and analysis were performed using the mgcv package of R Studio software (version 4.0.2 RStudio, PBC: Boston, MA, USA).
Results
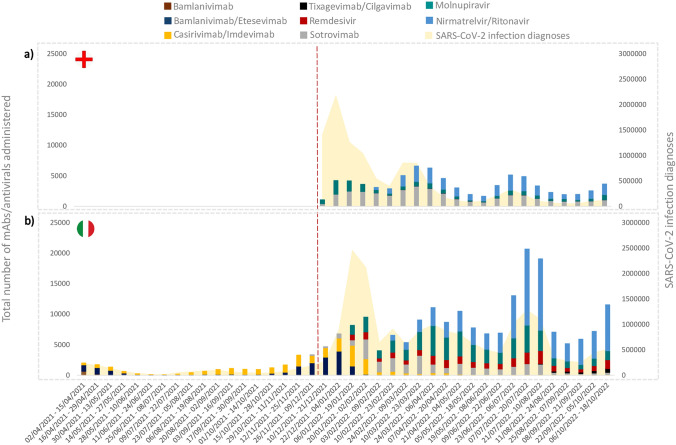
Overall, from December 2021 to October 2022, a total of 77,469 and 195,604 doses of mAbs/antivirals for early COVID-19 treatment were administered to a total of 10,630,903 (7.3 per 1000) and 18,168,365 (10.8 per 1000) patients who tested positive for SARS-CoV-2 infection in England and Italy, respectively [11, 12]. Considering the administered doses during the whole study period, sotrovimab (46.1%) and nirmatrelvir/ritonavir (34.1%) were the two most frequently administered drugs in England (Fig. 1A), while nirmatrelvir/ritonavir (38.6%) and molnupiravir (24.6%) in Italy (Fig. 1B).
Fig. 1.
Total number of mAb/antiviral doses administered in England (a) and Italy (b) for early treatment of COVID-19 outpatients from December 2021 to October 2022 in relation to the number of SARS-CoV-2 infection diagnoses in the same period. mAbs monoclonal antibodies, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2. The dotted line marks the start of the study period during which data were available for both England and Italy
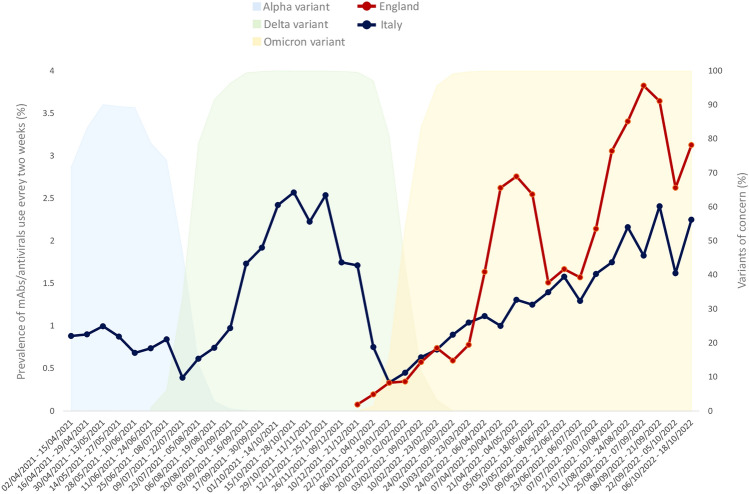
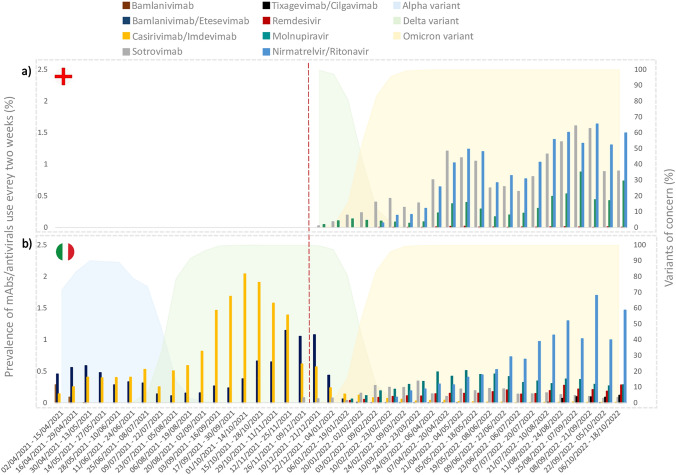
Overall, the prevalence of use of mAbs/antivirals as a whole every two weeks increased slowly from 0.07% to 3.1% in England (highest prevalence, 3.8%) and from 0.9% to 2.3% in Italy (highest prevalence, 2.6%) (Fig. 2). In England, the highest two weeks prevalence was reported for nirmatrelvir/ritonavir (1.6%), sotrovimab (1.6%) and molnupiravir (0.9%) (Fig. 3A), while for nirmatrelvir/ritonavir (1.7%) and molnupiravir (0.5%) in Italy (Fig. 3B). Compared with England, in Italy a lower prevalence of use for sotrovimab (0.3%) and a higher prevalence of use for remdesivir (0.3% versus 0.01%,) were observed. As for tixagevimab/cilgavimab, authorized only in Italy for COVID-19 therapeutic use, the highest prevalence of use every two weeks equal to 0.1% was observed in Italy.
Fig. 2.
Prevalence of use every 2 weeks of mAbs/antivirals as a whole for early treatment of COVID-19 outpatients based on the distribution of specific SARS-CoV-2 variants in England and Italy from December 2021 to October 2022. mAbs monoclonal antibodies
Fig. 3.
Prevalence (%) of use every two weeks of mAbs/antivirals by individual compound for early treatment of COVID-19 outpatients based on the distribution of specific SARS-CoV-2 variants in England (a) and Italy (b) from December 2021 to October 2022. mAbs monoclonal antibodies. The dotted line marks the start of the study period during which data were available for both England and Italy
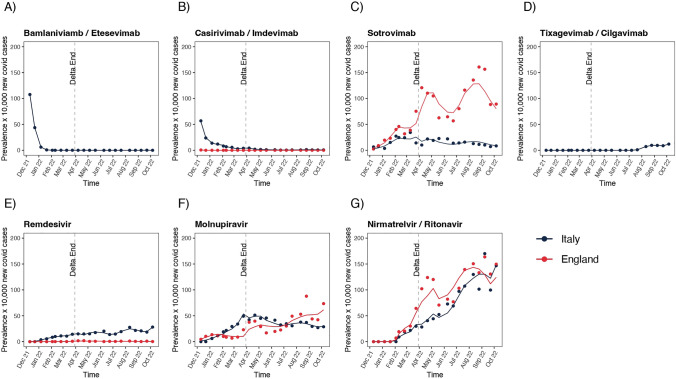
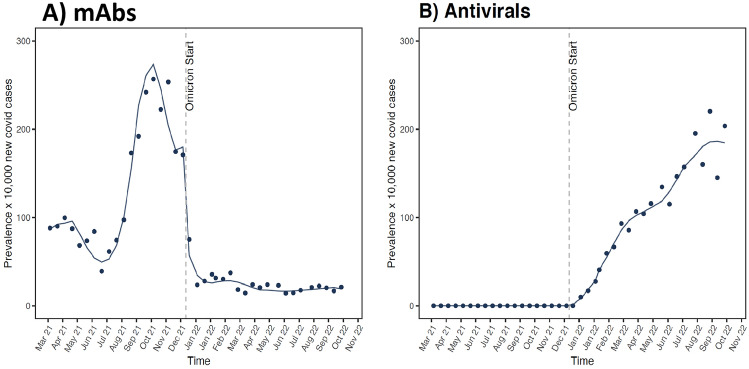
As reported in Fig. 4 and Table 1, the end of Delta SARS-CoV-2 variant predominance and the transition towards the Omicron predominance period was associated with a substantial increase in the use of sotrovimab, tixagevimab/cilgavimab, remdesivir, molnupiravir and nirmatrelvir/ritonavir and a reduction of other marketed monoclonal antibodies, such as bamlanivimab/etesevimab and casirivimab/imdevimab, in both England and Italy. Of note, on ITS analysis a steady increase of sotrovimab use in England after the end of the Delta variant (prevalence ratio, 4.4), and to a much lesser extent in Italy (prevalence ratio, 1.1), was observed. Concerning antivirals, an increase in the use of molnupiravir (prevalence ratio, 2.5) was observed in Italy, again to a lesser extent than in England (prevalence ratio, 4.2). Similarly, there was an increase in the use of remdesivir in Italy (prevalence ratio, 2.6) as well as in England, where the use of this drug in COVID-19 outpatients was, however, marginal. As for nirmatrelvir/ritonavir, a substantial increase in the prevalence of use was observed in both England and Italy with a prevalence ratio of 9.2 and 9.1, respectively.
Fig. 4.
Time series analysis of monoclonal antibody/antiviral use before and after the final period of Delta variant predominance in England and Italy
Table 1.
Interrupted time-series analysis of monoclonal antibodies and antivirals drugs use across two different time segments: pre- and post-end of Delta variant, in England and Italy
| Country | βb | Prevalence ratio | 95% CI | p-Value |
p-Value (Italy versus England) |
|
|---|---|---|---|---|---|---|
| Bamlaniviamb/etesevimaba | Italy | – | – | – | – | – |
| England | – | – | – | – | ||
| Sotrovimab | Italy | 0.080 | 1.083 | 1.052–1.116 | < 0.001 | < 0.001 |
| England | 1.491 | 4.445 | 4.322–4.572 | < 0.001 | ||
| Tixagevimab/cilgavimaba | Italy | – | – | – | – | – |
| England | – | – | – | – | ||
| Casirivimab/imdevimab | Italy | −1.630 | 0.195 | 0.180–0.212 | < 0.001 | – |
| Englanda | – | – | – | – | ||
| Remdesivir | Italy | 0.963 | 2.619 | 2.521–2.718 | < 0.001 | < 0.001 |
| England | 2.228 | 9.281 | 6.896–12.553 | < 0.001 | ||
| Molnupiravir | Italy | 0.931 | 2.537 | 2.479–2.596 | < 0.001 | < 0.001 |
| England | 1.448 | 4.254 | 4.100–4.410 | < 0.001 | ||
| Nirmatrelvir/ritonavir | Italy | 2.210 | 9.115 | 8.846–9.393 | < 0.001 | 0.440 |
| England | 2.220 | 9.207 | 8.935–9.487 | 0.010 |
CI confidence interval
aNot applicable
bLevel change parameter
In Italy, in the period April to December 2021 a total of 21,056 doses of mAbs/antivirals were administered, with casirivimab/imdevimab and bamlanivimab/etesevimab being the most widely used (Fig. 1B), with a total prevalence of use equal to 0.5% and 0.6%, respectively, during the alpha period (from April to mid-July 2021) (Fig. 3B). As for the Delta period (from mid-July to mid-December 2021), a prevalence of use of 2.0% and 1.1% was observed for casirivimab/imdevimab and bamlanivimab/etesevimab, respectively. In Fig. 5 and Table 2, the ITS analysis showed a peak for the prevalence of mAbs use in Italy in October 2021, which rapidly declined thereafter after the spread of the Omicron variant (Fig. 4A). In Fig. 4B, a huge increase of antivirals during the predominance of the Omicron variant is observed (13% increase every two weeks).
Fig. 5.
Time series analysis of mAb and antiviral use before and after the start of Omicron variant predominance in Italy. mAbs monoclonal antibodies
Table 2.
Interrupted time-series analysis of monoclonal antibodies and antivirals use across two different time segments: pre-Omicron and Omicron predominance in Italy
| Time window | β | Prevalence ratio | 95% CI | p-Value | |
|---|---|---|---|---|---|
| mAbs | Pre-omicrona | 0.008 | 1.008 | 1.006–1.009 | 0.192 |
| Omicronb | −0.601 | 0.548 | 0.518–0.577 | < 0.001 | |
| Omicrona | −0.024 | 0.976 | 0.973–0.979 | < 0.001 | |
| Antivirals | Omicronc, b | – | – | – | – |
| Omicrona | 0.121 | 1.129 | 1.100–1.158 | < 0.001 |
mAbs monoclonal antibodies, CI confidence interval
aSlope change parameter
bLevel change parameter
cNot applicable
Discussion
This is the first population-based study that analysed and compared the nationwide use of mAbs and antivirals for early COVID-19 treatment in outpatient setting during the period December 2021 to October 2022 in England versus Italy using publicly available data. A progressively increasing prevalence of use of mAbs and antivirals against SARS-CoV-2 in both Italy and England was observed. This finding was mostly determined by the marketing of the nirmatrelvir/ritonavir and molnupiravir as well as the extension of remdesivir use also for early treatment of COVID-19 outpatients, almost entirely replacing mAbs. In particular, in the most recent observation period, nirmatrelvir/ritonavir has been the most widely used antiviral, in line with the WHO clinical management of COVID-19 guidelines [20] as well as the European Society of Clinical Microbiology and Infectious Diseases guidelines [21]. A similar trend was observed in the USA, as reported on the US Department of Health & Human Services website [22]: in the period December 2021 to October 2022, mAb administration was replaced by nirmatrelvir/ritonavir and molnupiravir, due to the spread of new SARS-CoV-2 variants which were much less sensitive to mAbs anti-spike neutralization activity [23], in line with the National Institute of Health guidelines for COVID-19 treatment [24]. However, the prevalence of use of mAbs and antivirals is rather low as the highest reported prevalence of use within two weeks in patients diagnosed with SARS-CoV-2 infection was 3.8% in England and 2.6% in Italy. These findings are, however, explained by the fact that both mAbs and antivirals are approved specifically only for the early treatment of symptomatic adults or paediatric outpatients with specific comorbidities at high risk of developing severe COVID-19, which is only a minor proportion of all patients diagnosed with SARS-CoV-2 infection that were included as denominator in our study. On the other hand, as highlighted by Dal-Ré et al. [25], it was extremely challenging for all the national healthcare systems to be adequately adapted for the timely and correct use of these therapies as eligible patients have to be identified by general practitioners (GPs), referred to specialists in most cases for antiviral prescriptions and being dispensed and administered the drug within 5 days from the onset of symptoms. The availability for people who are eligible for treatment in association with the prescription process reflects several challenges for authorities. Therefore, the likely underutilization of mAbs and antivirals in at-risk patients with COVID-19 might be mainly due to a non-homogeneity of well-established networks between GPs (who identified and signalled the potential candidates to early treatment) and specialists (who confirmed the eligibility and prescribed the drug) causing a delay in access to treatment (Supplementary Table 2). Accordingly, in Italy we observed an increase in the use of nirmatrelvir/ritonavir since general practitioners have been directly authorized to prescribe this drug starting from 21 April 2022 [26]. Moreover, it is worth considering the method of administration of these new drugs, which also affects their use [4]. Monoclonal antibodies, as well as remdesivir, require intravenous (IV) infusion in healthcare facilities in which patients can be monitored during and for at least 1 h after administration (Supplementary Table 2). For this reason, in most cases nirmatrelvir/ritonavir is the preferred drug because of its oral administration, as well as its high efficacy [27]. However, the main disadvantage of nirmatrelvir/ritonavir is due to the high number of drug–drug interactions it has and therefore often cannot be administered.
Overall, our results showed that the increased use of mAbs during Delta predominance decreased with the spread of Omicron, while a more widespread use of sotrovimab, molnupiravir and nirmatrelvir/ritonavir was observed. This finding is consistent with the documented reduction of mAb efficacy against Omicron sub-lineages [23, 28] as it is known that mAbs act by binding to the SARS-CoV-2 spike protein, which varies according to the virus variant. On the contrary, antiviral therapies have a more unspecific mechanism of action which is unaffected by virus variants and the spike protein mutations. Several in vitro studies showed in fact that sotrovimab retained most of the activity against Omicron/BA.1 but was inhibited by Omicron/BA.2, while molnupiravir and nirmatrelvir/ritonavir consistently maintained in vitro activity against both BA.1 and BA.2 sub-lineages [29, 30]. However, despite the wide use of molnupiravir for early treatment of COVID-19 outpatients, on 24 February 2023, the EMA recommended against marketing authorization for molnupiravir for the failure to demonstrate a clinical benefit in terms of reduction of mortality, hospitalizations, duration of illness or time to recovery [8].
As for remdesivir, during the study period the public reports from England documented a much wider use in hospitalized patients (18,503 doses) than outpatients (203 doses) [10], as probably the result of its IV infusion for three consecutive days, as well as national health policies, while tixagevimab/cilgavimab in England is authorized only for COVID-19 prevention. Similarly, in Italy during the same observation period, overall higher use of remdesivir in hospitalized patients as compared with outpatients was reported, albeit with no major differences in terms of doses administered between the two settings (23,893 versus 21,402 doses) [31].
Similar findings were observed in a drug utilization study conducted in Scotland in the period between December 2021 and September 2022. Of 11,465 COIVD-19 outpatients, 47.4% were treated with nirmatrelvir/ritonavir, 26.7% with sotrovimab and 24.4% with molnupiravir [32].
The use of mAbs/antivirals for early treatment of patients diagnosed with SARS-CoV-2 infection varies between countries on the basis of different dates of approval and market availability. For instance, at the loco-regional level in Italy at the beginning of the Omicron wave, just before nirmatrelvir/ritonavir approval, a shortage of sotrovimab was experienced, and for this reason molnupiravir was mainly prescribed.
This study has several limitations that should be acknowledged. Only aggregated data were available in the public reports, thus not allowing stratification by gender, age and comorbidities to explore inequalities in prescribing across different patient categories. For the same reason, we could not identify specifically the patients with COVID-19 who were eligible for receiving the antiviral treatment. Therefore, for calculating the prevalence of mAb/antiviral use we considered as denominator all patients diagnosed with SARS-CoV-2 infection, including the asymptomatic ones, identified during the study period, even if only a portion of them were likely to be eligible for mAb/antiviral treatment. In addition, the number of positive tests is strictly dependent on the country-specific public health indications, including screening campaign in high-risk population. Indeed, a higher number of positive diagnostic tests was reported in Italy as compared with England during the study period. This difference may be due to the fact that free SARS-CoV-2 infection testing has been stopped in England since 1 April 2022 [33] and this probably affected the denominator used for calculating the prevalence of mAb/antiviral use. Accordingly, in England a substantial reduction in the number of diagnostic tests performed and, consequently, in the patients diagnosed with SARS-CoV-2 infection was observed in the period 1 April to 31 October 2022, as compared with the period 1 December to 31 March 2022 (average monthly performed positive tests: 2,258,790 versus 8,895,396) [11]. Free SARS-CoV-2 infection testing continued to be mostly warranted for symptomatic subjects in high-risk settings, and as such, this may have contributed to the observed increased prevalence of use of mAbs/antivirals in England versus Italy starting from 1 April 2022. On the other hand, SARS-CoV-2 infection-diagnosed outpatients enrolled in the ongoing clinical trials of antivirals were not included in the public reports. As the number of users enrolled in clinical trials may differ across countries, this may have further affect the comparison in prevalence of use of antivirals by country. As an example, in the UK the Panoramic trial enrolled > 25,000 COVID-19 outpatients [34] and the study start date is overlapping with that of our study.
Another potential limitation is that as the numerator we considered the number of administered doses (i.e. treatment cycles) instead of number of users. As for all mAbs and antivirals for early COVID-19 treatment in outpatient setting, only one treatment cycle is administered to each patient with COVID-19, and as there is limited opportunity for switching to other antivirals due to lack of efficacy, we can assume that number of administered doses corresponds to the number of mAb/antiviral users. On the other hand, the number of administered doses is expected to be reasonably accurate as special intensive registry-based monitoring for each approved antiviral for early COVID-19 treatment in Italy and in England was implemented.
Conclusions
In this dual nationwide study, the prevalence of use of mAbs/antivirals for early outpatient treatment in all patients diagnosed with SARS-CoV-2 infection increased from 0.07% to 3.1% in England and 0.9% to 2.3% in Italy from December 2021 to October 2022. This relative low prevalence of use is likely due to the limited eligibility for antiviral treatment that is restricted to only those at risk of severe COVID-19; however, underutilization of those drugs due to a non-homogeneity of well-established networks between GPs and specialists cannot be ruled out.
The trend of use of specific antiviral drugs for early treatment of COVID-19 outpatients varied over time in relation to the distribution of SARS-CoV-2 variants with some differences across countries. In accordance with the national guidelines and the recommendations of scientific societies for the management of mild-to-moderate COVID-19, in the most recent period nirmatrelvir/ritonavir was the most frequently prescribed antiviral.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement.
Conflicts of interest
Gianluca Trifirò has served in the past three years on advisory boards/seminars funded by SANOFI, Eli Lilly, AstraZeneca, Abbvie, Servier, Mylan, Gilead and Amgen; he was the scientific director of a Master program on pharmacovigilance, pharmacoepidemiology and real-world evidence which has received non-conditional grant from various pharmaceutical companies; he coordinated a pharmacoepidemiology team at the University of Messina until October 2020, which has received funding for conducting observational studies from various pharmaceutical companies (Boehringer Ingelheim, Daichii Sankyo, PTC Pharmaceuticals). He is also scientific coordinator of the academic spin-off “INSPIRE srl” which has received funding for conducting observational studies from contract research organizations (RTI Health Solutions, Pharmo Institute N.V.). All the above-mentioned activities are not related to the topic of the manuscript. Mariam Molokhia has received grants previously from the International Serious Adverse Events Consortium, SAEC (not related to the topic of the manuscript). The other authors have no conflict of interest to disclose.
Data availability
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Author contributions
All authors contributed equally to this work. All authors read and approved the final manuscript.
Code availability
Not applicable.
Footnotes
Francesco Ciccimarra and Nicoletta Luxi contributed equally to the paper as first authors.
References
- 1.Centre for Disease Control and Prevention (CDC). CDC Museum COVID-19 Timeline. 2023. Accessed March 6, 2023. Available from: https://www.cdc.gov/museum/timeline/covid19.html.
- 2.World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard. 2023. Accessed March 6, 2023. Available from: https://covid19.who.int.
- 3.Sultana J, Crisafulli S, Gabbay F, Lynn E, Shakir S, Trifirò G. Challenges for drug repurposing in the COVID-19 pandemic era. Front Pharmacol. 2020;11:588654. doi: 10.3389/fphar.2020.588654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Medicines Agency. COVID-19 treatments. 2023. Accessed March 6, 2023. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-treatments.
- 5.Italian Medicines Agency (AIFA). Use of oral antivirals for COVID-19. 2023. Accessed March 6, 2023. Available from: https://www.aifa.gov.it/en/uso-degli-antivirali-orali-per-covid-19.
- 6.Italian Medicines Agency (AIFA). Use of monoclonal antibodies for COVID-19. 2023. Accessed March 6, 2023. Available from: https://www.aifa.gov.it/en/uso-degli-anticorpi-monoclonali.
- 7.UK Parliament. Drug Therapies for COVID-19. 2023. Accessed March 6, 2023. Available from: https://post.parliament.uk/drug-therapies-for-covid-19/.
- 8.European Medicines Agency (EMA). Refusal of the marketing authorisation for Lagevrio (molnupiravir). 2023. Accessed March 6, 2023. Available from: https://www.ema.europa.eu/en/documents/smop-initial/questions-answers-refusal-marketing-authorisation-lagevrio-molnupiravir_en.pdf.
- 9.European Medicines Agency (EMA). ETF warns that monoclonal antibodies may not be effective against emerging strains of SARS-CoV-2. 2022. Accessed March 6, 2023. Available from: https://www.ema.europa.eu/en/news/etf-warns-monoclonal-antibodies-may-not-be-effective-against-emerging-strains-sars-cov-2#:~:text=Contacts-,ETF%20warns%20that%20monoclonal%20antibodies%20may%20not%20be%20effective,strains%20of%20SARS%2DCoV%2D2&text=EMA's%20Emergency%20Task%20Force%20(ETF,of%20SARS%2DCoV%2D2.
- 10.National Health Service (NHS) England. COVID-19 Therapeutics (antivirals, neutralising monoclonal antibodies and interleukin 6 inhibitors). 2023. Accessed March 6, 2023. Available from: https://www.england.nhs.uk/statistics/statistical-work-areas/covid-therapeutics-antivirals-and-neutralising-monoclonal-antibodies/.
- 11.GOV.UK Coronavirus (COVID-19) in the UK. Testing in England. 2023. Accessed March 6, 2023. Available from: https://coronavirus.data.gov.uk/details/testing?areaType=nation&areaName=England.
- 12.Italian National Institute of Health. COVID-19 integrated surveillance: key national data. 2023. Accessed March 6, 2023. Available from: https://www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza-dati.
- 13.European Centre for Disease Prevention and Control. Case definition for COVID-19, as of 3 December 2020. Accessed March 6, 2023. Available from: https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition.
- 14.European Centre for Disease and Control and World Health Organization. Joint ECDC-WHO Regional Office for Europe Weekly COVID-19 Surveillance Bulletin. 2023. Accessed January 12, 2023. Available from: https://worldhealthorg.shinyapps.io/euro-covid19/.
- 15.Schweizer ML, Braun BI, Milstone AM. Research methods in healthcare epidemiology and antimicrobial stewardship-quasi-experimental designs. Infect Control Hosp Epidemiol. 2016;37(10):1135–1140. doi: 10.1017/ice.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 17.Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38–S44. doi: 10.1016/j.acap.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Kontopantelis E, Doran T, Springate DA, Buchan I, Reeves D. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ. 2015;350:2750. doi: 10.1136/bmj.h2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonazzo IC, Fornari C, Maumus-Robert S, et al. Impact of COVID-19 Lockdown, during the two waves, on drug use and emergency department access in people with epilepsy: an interrupted time-series analysis. Int J Environ Res Public Health. 2021;18(24):13253. doi: 10.3390/ijerph182413253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO). Clinical management of COVID-19: living guideline, 15 September 2022. Accessed March 6, 2023. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2023.1. [PubMed]
- 21.Bartoletti M, Azap O, Barac A, Bussini L, Ergonul O, Krause R, Martin-Quiros A, Paño-Pardo JR, Power N, Sibani M, Szabo BG, Tsiodras S, Zollner-Schwetz I, Rodríguez-Baño J. European Society of Clinical Microbiology and Infectious Diseases guidelines for coronavirus disease 2019: an update on treatment of patients with mild/moderate disease. Clin Microbiol Infect. 2022;28(12):1578–1590. doi: 10.1016/j.cmi.2022.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. Department of Health & Human Services. COVID-19 Therapeutics Thresholds, Orders, and Replenishment by Jurisdiction. 2023. Accessed March 6, 2023. Available from: https://aspr.hhs.gov/COVID-19/Therapeutics/orders/Pages/default.aspx.
- 23.Takashita E, Yamayoshi S, Simon V, van Bakel H, Sordillo EM, Pekosz A, Fukushi S, Suzuki T, Maeda K, Halfmann P, Sakai-Tagawa Y, Ito M, Watanabe S, Imai M, Hasegawa H, Kawaoka Y. Efficacy of antibodies and antiviral drugs against Omicron BA.2.12.1, BA.4, and BA.5 subvariants. N Engl J Med. 2022;387(5):468–470. doi: 10.1056/NEJMc2207519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institute of health (NIH) COVID-19 Treatment Guidelines. Table 2a. Therapeutic Management of Non hospitalized Adults With COVID-19. 2022. Accessed March 6, 2023. Available from: https://www.covid19treatmentguidelines.nih.gov/tables/management-of-nonhospitalized-adults-summary/.
- 25.Dal-Ré R, Becker SL, Bottieau E, Holm S. Availability of oral antivirals against SARS-CoV-2 infection and the requirement for an ethical prescribing approach. Lancet Infect Dis. 2022;22(8):e231–e238. doi: 10.1016/S1473-3099(22)00119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Italian Medicines Agency (AIFA). Prescription of Paxlovid by the General Practitioner. 2022. Accessed March 6, 2023. Available from: https://www.aifa.gov.it/en/-/prescrizione -paxlovid-mmg.
- 27.Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, Baniecki M, Hendrick VM, Damle B, Simón-Campos A, Pypstra R, Rusnak JM. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Focosi D, McConnell S, Casadevall A, Cappello E, Valdiserra G, Tuccori M. Monoclonal antibody therapies against SARS-CoV-2. Lancet Infect Dis. 2022;22(11):e311–e326. doi: 10.1016/S1473-3099(22)00311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox M, Peacock TP, Harvey WT, et al. SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies. Nat Rev Microbiol. 2023;21(2):112–124. doi: 10.1038/s41579-022-00809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vangeel L, Chiu W, De Jonghe S, et al. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antiviral Res. 2022;198:105252. doi: 10.1016/j.antiviral.2022.105252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Italian Medicines Agency (AIFA). Monitoring of antivirals for COVID-19: new report published. 2022. Accessed March 6, 2023. Available from: https://www.aifa.gov.it/en/-/monitoraggio-antivirali-per-covid-19-pubblicato-il-report-n.-21.
- 32.Tibble H, Mueller T, Proud E, Hall E, Kurdi A, Robertson C, Bennie M, Woolford L, Sheikh A. Uptake of monoclonal antibodies and antiviral therapies for COVID-19 in Scotland. Lancet. 2023;401(10371):101–102. doi: 10.1016/S0140-6736(22)02398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.GOV.UK. Changes to COVID-19 testing in England from 1 April. 2022. Accessed March 6, 2023. Available from: https://www.gov.uk/government/news/changes-to-covid-19-testing-in-england-from-1-april.
- 34.Butler CC, Hobbs FDR, Gbinigie OA, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial [published online ahead of print, 2022 Dec 22]. Lancet. 2022. doi:10.1016/S0140-6736(22)02597-1 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.