Abstract
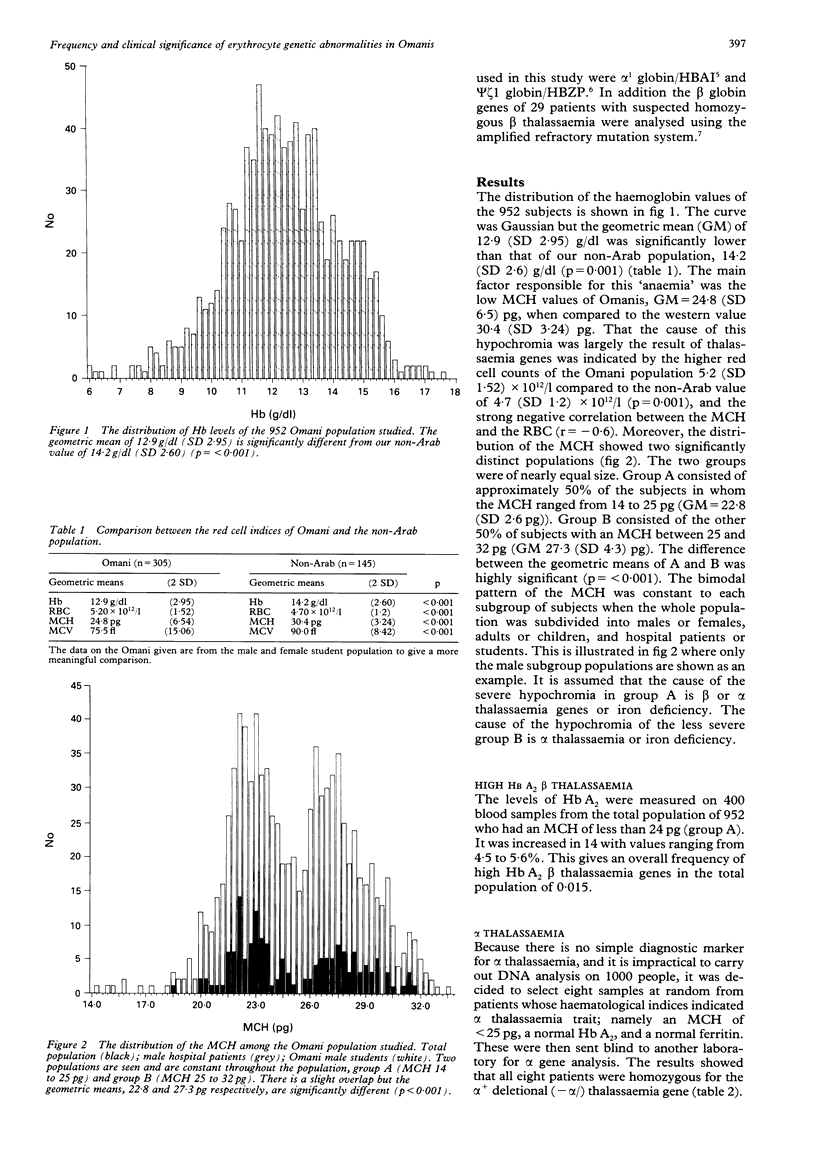
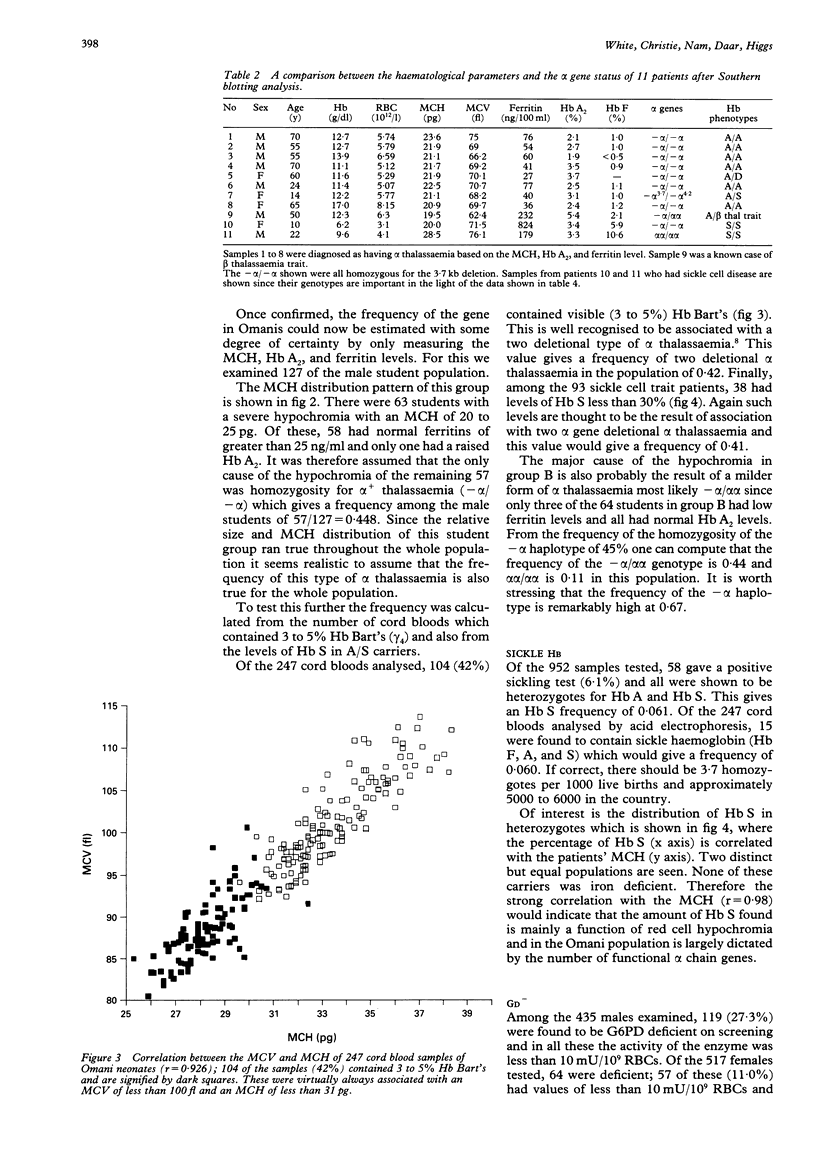
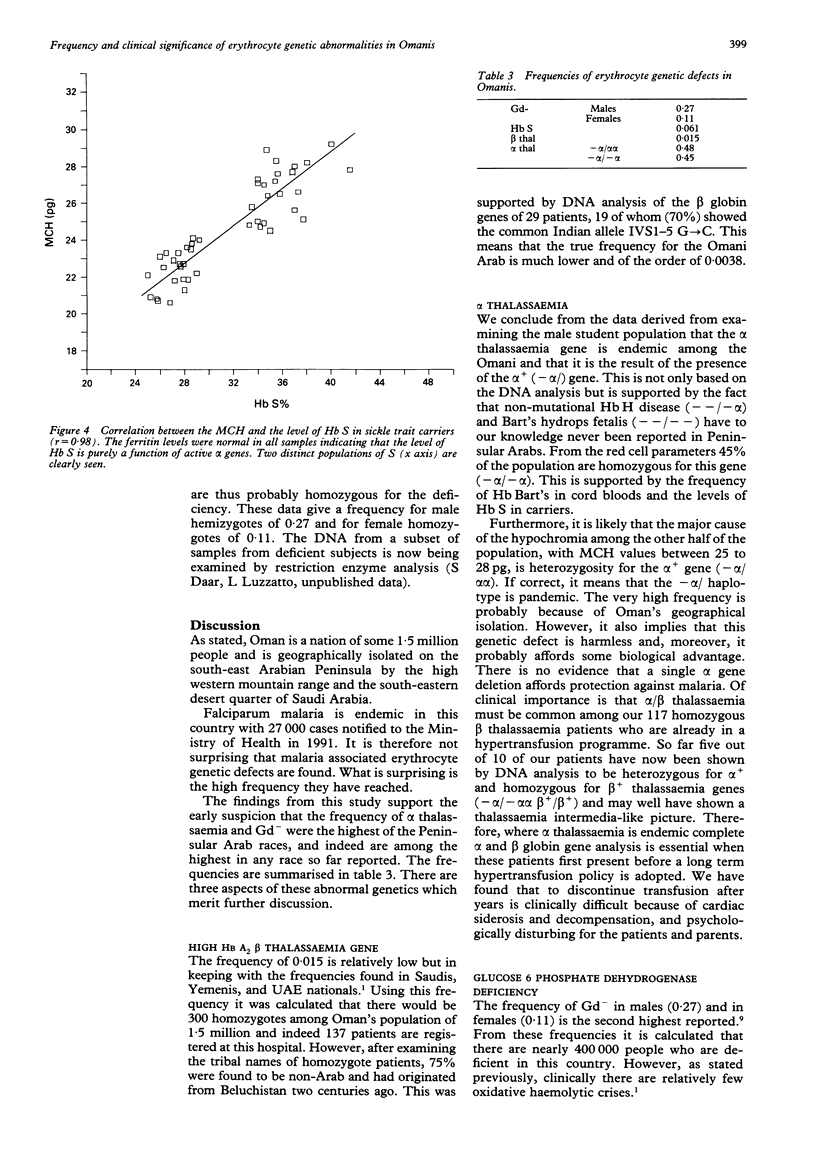
The frequencies of four malaria associated erythrocyte genetic abnormalities have been established in 1000 Omani subjects. They are: homozygous alpha+ thalassaemia (-alpha/-alpha) 0.45; high Hb A2 beta thalassaemia trait 0.015; sickle trait (Hb A/S) 0.061; and glucose 6 phosphate dehydrogenase deficiency (Gd-): males 0.27, females 0.11. From our data the alpha+ (-alpha/) thal gene (confirmed by Southern blotting) is pandemic in this population. Moreover, in spite of the very high frequency of Gd-, oxidative haemolytic syndromes are very uncommon. Also preliminary data indicate that among the Omani population with sickle cell disease, homozygosity of the alpha+ gene markedly modifies the clinical picture.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler E. Glucose-6-phosphate dehydrogenase deficiency. N Engl J Med. 1991 Jan 17;324(3):169–174. doi: 10.1056/NEJM199101173240306. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Kurdi-Haidar B., Mason P. J., Berrebi A., Ankra-Badu G., al-Ali A., Oppenheim A., Luzzatto L. Origin and spread of the glucose-6-phosphate dehydrogenase variant (G6PD-Mediterranean) in the Middle East. Am J Hum Genet. 1990 Dec;47(6):1013–1019. [PMC free article] [PubMed] [Google Scholar]
- Lauer J., Shen C. K., Maniatis T. The chromosomal arrangement of human alpha-like globin genes: sequence homology and alpha-globin gene deletions. Cell. 1980 May;20(1):119–130. doi: 10.1016/0092-8674(80)90240-8. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Gil A., Maniatis T. The structure of the human zeta-globin gene and a closely linked, nearly identical pseudogene. Cell. 1982 Dec;31(3 Pt 2):553–563. doi: 10.1016/0092-8674(82)90311-7. [DOI] [PubMed] [Google Scholar]
- Varawalla N. Y., Old J. M., Sarkar R., Venkatesan R., Weatherall D. J. The spectrum of beta-thalassaemia mutations on the Indian subcontinent: the basis for prenatal diagnosis. Br J Haematol. 1991 Jun;78(2):242–247. doi: 10.1111/j.1365-2141.1991.tb04423.x. [DOI] [PubMed] [Google Scholar]
- White J. M., Byrne M., Richards R., Buchanan T., Katsoulis E., Weerasingh K. Red cell genetic abnormalities in Peninsular Arabs: sickle haemoglobin, G6PD deficiency, and alpha and beta thalassaemia. J Med Genet. 1986 Jun;23(3):245–251. doi: 10.1136/jmg.23.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]


