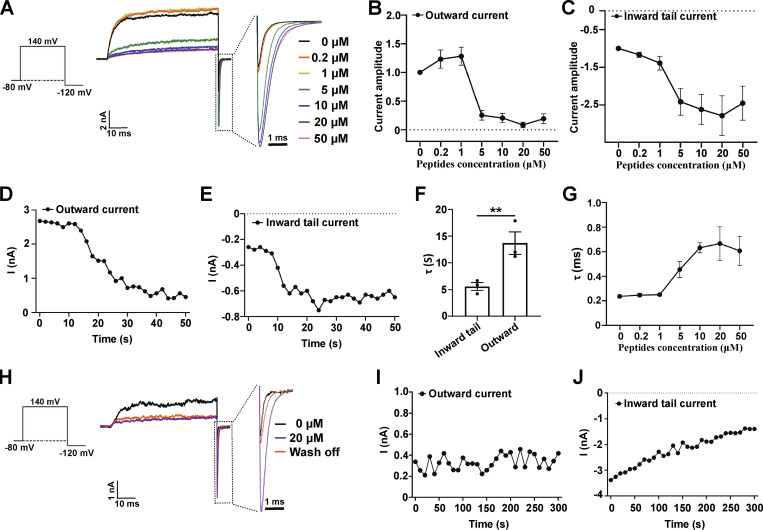
Figure 2.
Effects of a synthetic peptide mimic of the γ1 subunit’s C-terminal positively charged region on BKα channels. (A) The effect of different concentrations of γ1 peptide on BKα channel currents elicited by activation at 140 mV and then deactivation at −120 mV. The current data were low-pass filtered at 10 kHz. (B) Normalized outward current amplitudes of BKα channels in the presence of different concentrations of the γ1 peptide. (C) Normalized inward tail current amplitudes of BKα channels in the presence of different concentrations of the γ1 peptide. (D and E) Representative time-dependence of the maximal amplitude of the BKα channel outward currents (140 mV; D) and inward tail currents (−120 mV; E) upon application of 5 µM γ1 peptide. (F) Averaged rates (τ) of the peptide-induced changes in BKα channel inward tail currents and outward currents as shown in D and E. Unpaired Student’s t test (two-tailed) was used to calculate P values. ** is for P values ≤ 0.01 (P = 0.0073). n = 3. (G) The effect of different concentrations of the γ1 peptide on the averaged kinetics (τ) of BKα channel deactivation (inward tail currents) at −120 mV. (H) The effect of peptide wash-off on the BKα channel currents was elicited by activation at 140 mV and then deactivation at −120 mV. The γ1 peptide was applied at 20 µM for 1 min and then washed for 5 min. The current data were low-pass filtered at 10 kHz. (I and J) Representative time-dependence of the maximal amplitude of the BKα channel outward currents (140 mV; I) and inward tail currents (−120 mV; J) upon solution perfusion without peptide. The excised patches were pretreated (perfusion) with 20 µM γ1 peptide for 1 min. All BK channel currents were recorded in the virtual absence of [Ca2+]i. Error bars represent ± SEM.