Fig. 1. Mechanisms to maintain potassium (K) homeostasis after a K-rich meal.
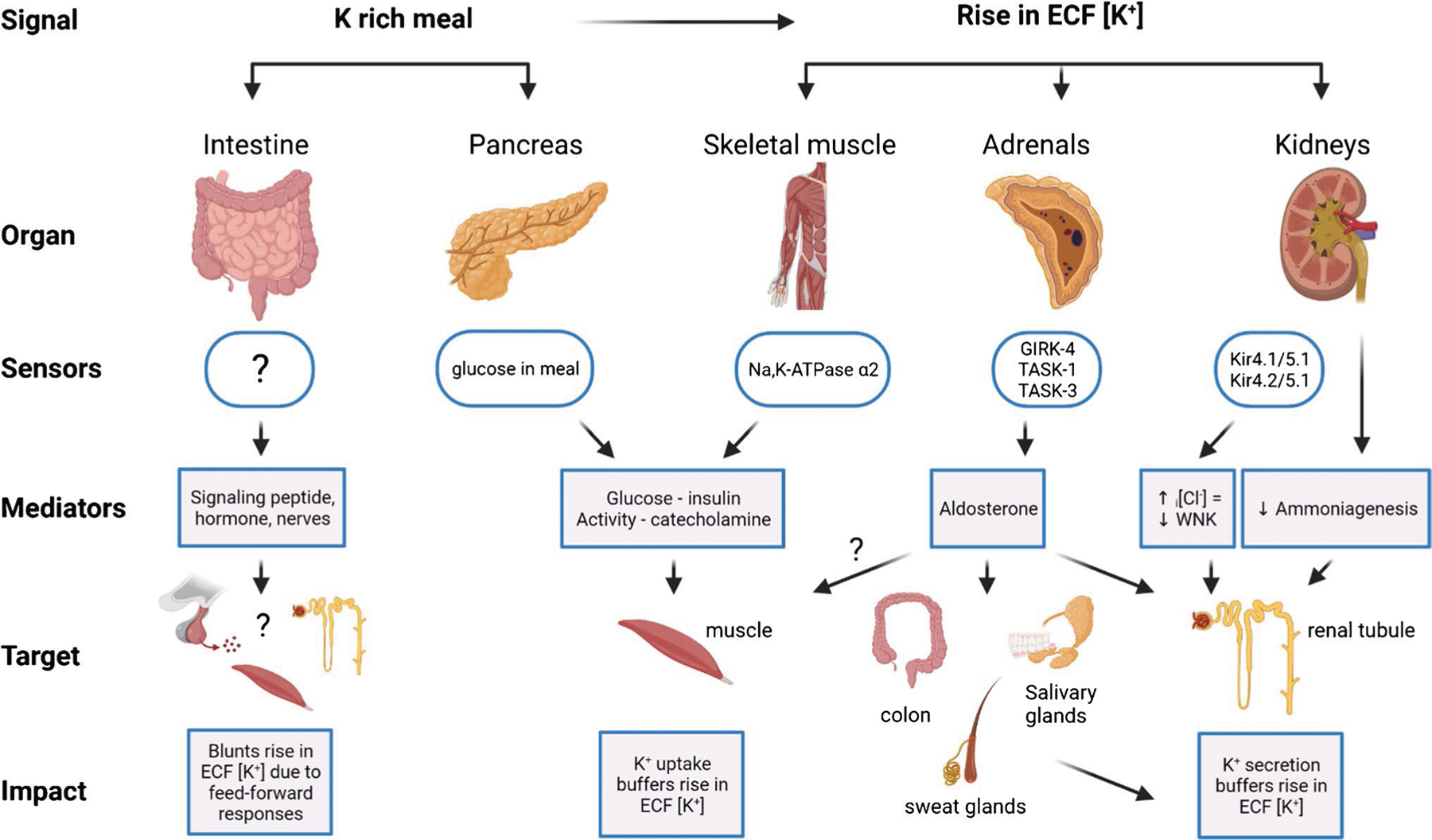
A K-rich meal stimulates the intestine to release an unidentified “gut factor” that potentially targets the pituitary to release a mediator that targets skeletal muscle and liver to take up K from ECF to ICF, in addition to stimulating the renal nephron to excrete K before a rise in plasma [K]. Additionally, the meal stimulates the pancreas to release insulin, a mediator that activates muscle Na,K-ATPase independent of a rise in plasma [K]. These feedforward mechanisms are the initial homeostatic response to buffering ECF [K] in response to K intake. As the meal is absorbed, the rise in plasma [K] is a power signal that targets (1) Skeletal muscle Na,K-ATPase alpha 2 isoform, which is kinetically activated by rising [K], (2) Kidney basolateral membrane K-channel sensors Kir4.1/5.1 and potentially Kir4.2/5.1, which rapidly stimulate signaling cascades that mediate increased K secretion and excretion and depress ammoniagenesis, and (3) Adrenal glomerulosa cell K channels GIRK-4, TASK-1, and TASK-3 which change membrane potential and cell [Ca] and stimulate biosynthesis and release of the mediator aldosterone. Aldosterone targets mineralocorticoid receptors in the renal distal nephron, colon, sweat and salivary glands to secrete and excrete K. Together, stimulation of K secretion and excretion match K output to input, forming complex feedforward plus feedback loops that are needed to tightly maintain ECF [K]. Figure created with BioRender.com
