Figure 6. An enhanced affinity of Hspa8G470R for synaptic co-chaperone proteins.
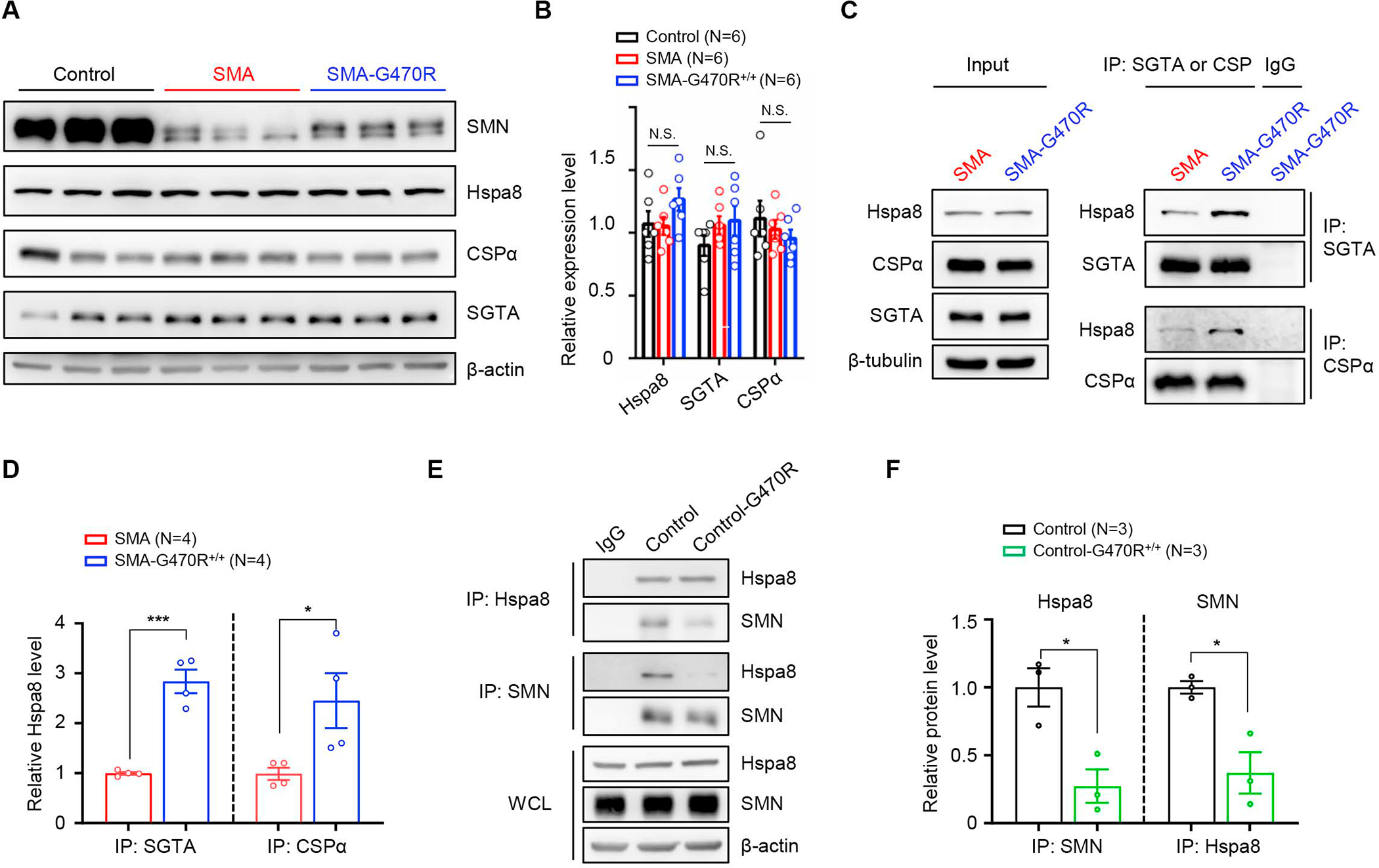
(A) Western blot analysis depicting equivalent levels of Hspa8 and other constituent members of a synaptic chaperone complex in PND9 brain tissue of controls and SMA mutants expressing WT or the G470R Hspa8 variant. (B) Quantified results of blot; N.S. – not significant, one-way ANOVA. (C) Co-immunoprecipitation (co-IP) analysis of relative affinities of WT Hspa8 or the G470R variant for its co-chaperones, SGTA and CSPα; the variant binds better to SGTA and CSPα. (D) Graph depicting the affinities of Hspa8WT and Hspa8G470R for their interacting partners. (E) Reciprocal co-IP analysis of brain-derived Hspa8 and SMN illustrates that the two interact and that there is weakened affinity of the G470R variant for SMN. (F) Quantification of relative affinities of WT Hspa8 or the G470R variant for SMN. Note: *, ***, P < 0.05, P < 0.001 respectively, t tests for analyses in panels D and F; brain lysates from PND9 mice were used for co-IP experiments. Data: mean ± SEM. See also Fig. S7.
