Abstract
Background
Smoking bans or restrictions can assist in eliminating nonsmokers' exposure to the dangers of secondhand smoke and can reduce tobacco consumption amongst smokers themselves. Evidence exists identifying the impact of tobacco control regulations and interventions implemented in general workplaces and at an individual level. However, it is important that we also review the evidence for smoking bans at a meso‐ or organisational level, to identify their impact on reducing the burden of exposure to tobacco smoke. Our review assesses evidence for meso‐ or organisational‐level tobacco control bans or policies in a number of specialist settings, including public healthcare facilities, higher education and correctional facilities.
Objectives
To assess the extent to which institutional smoking bans may reduce passive smoke exposure and active smoking, and affect other health‐related outcomes.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE, EMBASE, and the reference lists of identified studies. We contacted authors to identify completed or ongoing studies eligible for inclusion in this review. We also checked websites of state agencies and organisations, such as trial registries. Date of latest searches was 22nd June 2015.
Selection criteria
We considered studies that reported the effects of tobacco bans or policies, whether complete or partial, on reducing secondhand smoke exposure, tobacco consumption, smoking prevalence and other health outcomes, in public healthcare, higher educational and correctional facilities, from 2005 onwards.
The minimum standard for inclusion was having a settings‐level policy or ban implemented in the study, and a minimum of six months follow‐up for measures of smoking behaviour. We included quasi‐experimental studies (i.e. controlled before‐and‐after studies), interrupted time series as defined by the Cochrane Effective Practice and Organization of Care Group, and uncontrolled pre‐ and post‐ban data.
Data collection and analysis
Two or more review authors independently assessed studies for inclusion in the review. Due to variation in the measurement of outcomes we did not conduct a meta‐analysis for all of the studies included in this review, but carried out a Mantel‐Haenszel fixed‐effect meta‐analysis, pooling 11 of the included studies. We evaluated all studies using a qualitative narrative synthesis.
Main results
We included 17 observational studies in this review. We found no randomized controlled trials. Twelve studies are based in hospitals, three in prisons and two in universities. Three studies used a controlled before‐and‐after design, with another site used for comparison. The remaining 14 studies used an uncontrolled before‐and‐after study design. Five studies reported evidence from two participant groups, including staff and either patients or prisoners (depending on specialist setting), with the 12 remaining studies investigating only one participant group.
The four studies (two in prisons, two in hospitals) providing health outcomes data reported an effect of reduced secondhand smoke exposure and reduced mortality associated with smoking‐related illnesses. No studies included in the review measured cotinine levels to validate secondhand smoke exposure. Eleven studies reporting active smoking rates with 12,485 participants available for pooling, but with substantial evidence of statistical heterogeneity (I² = 72%). Heterogeneity was lower in subgroups defined by setting, and provided evidence for an effect of tobacco bans on reducing active smoking rates. An analysis exploring heterogeneity within hospital settings showed evidence of an effect on reducing active smoking rates in both staff (risk ratio (RR) 0.71, 95% confidence interval ( CI) 0.64 to 0.78) and patients (RR 0.86, 95% CI 0.76 to 0.98), but heterogeneity remained in the staff subgroup (I² = 76%). In prisons, despite evidence of reduced mortality associated with smoking‐related illnesses in two studies, there was no evidence of effect on active smoking rates (1 study, RR 0.99, 95% CI 0.84 to 1.16).
We judged the quality of the evidence to be low, using the GRADE approach, as the included studies are all observational.
Authors' conclusions
We found evidence of an effect of settings‐based smoking policies on reducing smoking rates in hospitals and universities. In prisons, reduced mortality rates and reduced exposure to secondhand smoke were reported. However, we rated the evidence base as low quality. We therefore need more robust studies assessing the evidence for smoking bans and policies in these important specialist settings.
Plain language summary
Do smoking bans at an institutional level help to stop people smoking?
Since some countries banned smoking in public places in 2004, there has been a reduction in secondhand smoke exposure (being affected by smoke from other people's cigarettes), and health has improved for smokers and nonsmokers. Being exposed to secondhand smoke can increase the chances of illness and death, and so a number of international health organisations support the introduction of methods to reduce exposure to tobacco and secondhand smoke, including smoking bans.
Studies have shown that workplaces providing services to help smokers to stop smoking have been effective. Services can include providing nicotine replacement therapy (NRT) and counselling support to help smokers quit. However, it is not known if policies that stop people smoking in institutions are effective. Whilst smoking is banned in many public places, it is not banned in all of them. Smoking is allowed in some healthcare organisations, universities and prisons.
Study characteristics
We searched for studies that measured whether introducing a smoking policy or ban, in hospitals, universities or prisons, reduced secondhand smoke exposure and helped people to quit smoking. The study could be in any language. It had to report information on health and smoking before the policy or ban started and for at least six months afterwards. We have included 17 studies in this review. Twelve studies provide evidence from hospitals, three from prisons and two from universities. The evidence is up‐to‐date to June 2015.
Key results
We grouped together 11 of the included studies, involving 12,485 people, and found that banning smoking in hospitals and universities increased the number of smoking quit attempts and reduced the number of people smoking. In prisons, there was a reduction in the number of people who died from diseases related to smoking and a reduction in exposure to secondhand smoke after policies and bans were introduced, but there was no evidence of reduced smoking rates.
Quality of the evidence
We found no relevant high‐quality studies to include in our review. Future high‐quality research may lead to a change in these conclusions and it is not possible to draw firm conclusions from the current evidence. We need more research from larger studies to investigate smoking bans and policies in these important settings.
Summary of findings
Summary of findings for the main comparison. Impact of institutional bans in hospitals, universities and prisons.
| Smoking rates and smoking‐related mortality, pre‐ and post‐smoking ban/policy change | ||||||
| Patient or population: Smokers Settings: Hospitals, universities, prisons Intervention: Introduction of smoking ban | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No policy | Active smoking rates after policy | |||||
| Active smoking, hospital setting | Study population | RR 0.75 (0.69 to 0.81) | 5986 (8 studies1,2) | ⊕⊕⊝⊝ low1,2 | ||
| 335 per 1000 | 251 per 1000 (231 to 271) | |||||
| Active smoking, university setting | Study population | RR 0.72 (0.64 to 0.80) | 6369 (2 studies1) | ⊕⊕⊝⊝ low1 | ||
| 194 per 1000 | 140 per 1000 (124 to 155) | |||||
| Active smoking, prison setting | Study population | RR 0.99 (0.84 to 1.16) | 130 (1 study3,4) | ⊕⊕⊝⊝ low3,4 | ||
| 829 per 1000 | 820 per 1000 (696 to 961) | |||||
| Smoking‐related mortality ‐ prison setting | Study population | Not estimable | 0 (2 studies) | Reductions in mortality for smoking‐related diseases noted in 2 studies (Binswanger 2014; Dickert 2015) after prisons adopted no‐smoking policies. | ||
| See comment | See comment | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1No control group 2Inconsistencies in staff and patient outcomes 3Inconsistencies in enforcement 4One study
Background
Health effects of smoking and exposure to second‐hand tobacco smoke
Over five million deaths worldwide are attributable to smoking, with exposure to secondhand tobacco smoke responsible for 600,000 deaths annually (WHO 2009; WHO 2013a). There is no safe level of exposure to secondhand smoke (SHS) (US Department of Health and Human Services 2014); however, in 2009 the World Health Organization (WHO) identified only a minority of countries worldwide that had implemented measures demanded in the Framework Convention on Tobacco Control (WHO 2009), with increasing implementation of measures in the intervening years (WHO 2014). One of these measures is a smoke‐free environment with specific legislation, with voluntary bans identified as inappropriate and unacceptable (WHO 2009).
Description of the intervention
Implementing bans at the institutional level
By the 1970s, the WHO had identified health as a fundamental human right (WHO 1978), and with the Ottawa Charter outlined key principles of health promotion advocating the settings approach (WHO 1986), including schools, workplaces, hospitals, prisons and cities. In the intervening years, the WHO has continued to voice the need for public health policies as a key requirement for promoting health, most recently in 2013 (WHO 2013b). A body of evidence exists highlighting the impact of tobacco control regulations and interventions in workplaces in general and at the individual level. Smoking bans or restrictions can assist in eliminating nonsmokers' exposure to the dangers of secondhand smoke and can reduce tobacco consumption amongst smokers themselves.
Baric 1993 identified a number of conditions necessary to achieve health promotion in a particular setting, including a healthy environment, integration of health promotion in daily activities, and creating conditions for reaching out to the community (Green 2015). To facilitate development of public health and health promotion activities, a systems‐based framework approach enables the development of initiatives across three strata or levels of health promotion, i.e. micro‐, meso‐ and macro‐ (WHO 2002). The levels influence each other and when functioning together can provide successful public health initiatives.
Micro‐interventions target the level of the individual, and there is evidence within tobacco control health promotion of the impact of behavioural interventions to reduce consumption and increase quit rates (Rigotti 2012; Stanton 2013). Meso‐level interventions, with which our review is concerned, operate at the level of organisation and community settings (WHO 2002). There is evidence from tobacco control initiatives for the impact of interventions at meso‐level, including schools (Coppo 2014; Thomas 2013), and general workplaces (Cahill 2014; IARC 2008, IARC 2009; Tan 2012). Evidence from community‐level initiatives is limited, and requires further research (Carson 2011). A recent review of policy‐level interventions and their impact on smoke exposure for smokers and nonsmokers provides examples of macro‐level interventions, or legislative frameworks, for worldwide health promotion (Frazer 2016; WHO 2002).
Why it is important to do this review
The evidence base has increased markedly since the first publication of the legislative bans review (Callinan 2010) and the recent update (Frazer 2016). Ongoing additional reports support the improved health outcomes associated with smoke‐free legislation (Been 2014; Kelleher 2014; Lee 2014). The Framework Convention on Tobacco Control's efforts to reduce tobacco consumption globally since 2003 have resulted in international support and an increase in anti‐smoking legislative actions (WHO 2008; WHO 2009; WHO 2014). The 2008 MPOWER evidence‐based measures included protection from tobacco smoke in the international fight to reduce the burden of tobacco‐related mortality and morbidity (WHO 2009; WHO 2013a). Whilst Frazer 2016 has identified the effectiveness of macro‐level bans, it is essential that we review the evidence for smoking bans at a meso‐level, to identify their impact on reducing the burden of exposure to tobacco smoke. We therefore examine the available literature on bans in specialist settings, specifically in public healthcare facilities, in higher educational and in correctional facilities, to identify the impact of such bans, whether complete or partial, on reducing smoke exposure, tobacco consumption, smoking prevalence and health outcomes.
Objectives
To assess the extent to which institutional smoking bans may reduce passive smoke exposure and active smoking, and affect other health‐related outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs), non‐RCTs (where investigators assign groups to conditions in a non‐random manner), controlled before‐and‐after studies (where allocation to different comparison conditions is not made by the investigators) and interrupted time series (where data are collected at multiple time points before and after an intervention to detect whether the intervention had a significantly greater effect than any underlying secular trend), as defined by the Cochrane Effective Practice and Organization of Care (EPOC) Group. We also considered uncontrolled before‐and‐after studies (comparing outcomes in the same participants or setting before and after implementation of the intervention). We required a minimum of six months follow‐up for inclusion.
Types of participants
We included bans in specialist settings, including healthcare facilities (hospitals, healthcare premises, residential homes), higher education, and correctional facilities (prisons and military institutions), where partial or complete indoor smoking bans or policies have been implemented. These specialist settings are included as smoking was not entirely banned or restricted with the introduction of national smoke‐free legislation in many jurisdictions.
Although they fit our definition of specialist settings, we did not include general workplaces or schools, as these have been reviewed previously (Cahill 2014; Coppo 2014; Fichtenberg 2002; IARC 2009; Thomas 2013). We did not include cars or recreational play areas.
We included participants within the specialist settings, whether smoker or nonsmoker.
Types of interventions
To be included in this review, the study must identify a partial or complete indoor smoking ban or policy in the specified settings. Studies were limited to those emerging since the introduction of the first Public Health (Tobacco) Act, prohibiting smoking in workplaces (GOI 2004), and following the implementation of the Treaty on Framework Convention on Tobacco Control in 2005 (WHO 2003). A reason for giving preference to studies with a background national smoke‐free ban (including state or regional bans) is robustly policy‐based. Many bans were put in place in public areas first, now progressing to bans in specialist settings. We can then compare and contrast studies with and without a national ban.
Types of outcome measures
Primary outcomes
The primary outcome measures were the impact of indoor smoking bans or policies in specialist settings on protection from passive smoke exposure or health‐related outcomes, or both. In order to examine sustained impact we required studies which reported baseline data and outcomes for at least six months after the introduction of the indoor smoking ban. Implementation of health promotion initiatives is challenging; previous research identified the need for a data collection period of a minimum of six months to one year, and up to two years for evaluating maintenance, at individual and organizational levels (Glasgow 1999; Green 2006). Sustainability of interventions at the settings level is essential (Glasgow 2006).
To assess passive smoke exposure, we preferred either biochemical confirmation of exposure to environmental tobacco smoke, with biological indicators in people such as cotinine or carbon monoxide measures, or information on health impacts, including hospital admission rates for conditions known to be related to smoke exposure, or both types of measure.
Secondary outcomes
We assessed active smoking outcomes, including reported smoking rates in the exposed or target population, and evidence of smoking cessation or quit attempts. We preferred studies that reported biochemically‐validated data on smoking cessation, as with passive smoke exposure.
Search methods for identification of studies
Search strategies comprised search terms both for key words and controlled‐vocabulary search terms for MEDLINE (MeSH) and EMBASE (EMTREE) related to indoor smoking bans as listed in (but not limited to) Appendix 1.
Electronic searches
We searched the following databases in June 2015:
the Cochrane Central Register of Controlled Trials (CENTRAL), 2015 Issue 7 (via CRSO);
MEDLINE to June (week 2) 2015 (via OVID);
MEDLINE in progress 15th June 2015 (via OVID)
EMBASE to 2015 week 24 (via OVID).
We limited the searches to studies from 2005 to the present. We searched reference lists of identified studies and contacted authors and relevant organizations for further information as necessary. We did not restrict eligibility based on language of publication.
Searching other resources
We searched Google Scholar using the term 'smoke ban' in July 2015. We searched Nicotine & Tobacco Research and tobacco addiction conference abstracts, and identified studies through personal communication with experts in the field. We checked websites of state agencies and organizations to identify further studies and reports.
Data collection and analysis
Selection of studies
The review process consisted of the following stages.
One review author (KF) downloaded eligible abstracts and titles into a reference management database with duplicate citations deleted.
One review author (JMcH) reviewed abstracts and titles to identify potentially eligible studies and obtained full‐text copies of these studies. A second review author (KF) independently reviewed all titles and abstracts from the main search strategy.
We made our final decision on eligibility based on the full text. Two review authors (KF, JMcH) independently extracted data from the included studies and compared results prior to entry into Review Manager 5 software (RevMan).
We resolved eligibility disagreements by discussion, by contacting study authors and by inviting a third review author (CK) to act as independent arbiter.
We recorded reasons for the exclusion of studies.
Data extraction and management
We used a data extraction form in this review adapted from one previously used in a similar review of national legislative smoking bans (Callinan 2010; Frazer 2016). One review author (KF) was responsible for entering all data into RevMan, Cochrane's statistical software. We recorded all decisions on the data extraction forms. A second review author (JMcH) checked the contents of the review.
We extracted the following data.
Title/unique identifier
Lead author of publication
Date of publication/report
Identification of data extractor
Country
Study setting
Description of intervention
Size of eligible population
Number of participants
Demographic characteristics of participants
Definition of abstinence and smoking status
Definition of exposure to secondhand smoke
Biochemical validation
Outcomes and how they were measured, including quit rates, acceptability
Length of follow‐up
Handling of dropouts and losses to follow‐up
Adverse effects of the intervention
Sources of funding
Potential conflicts of interest of the study authors
If studies were reported in more than one publication we extracted data from all publications onto one form so they are combined for reporting.
Assessment of risk of bias in included studies
Two review authors (KF, JMcH) independently assessed the risk of bias of the included studies, with disagreements resolved by discussion, and by consulting a third review author (CK). We assessed risk of bias using criteria in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and assigned judgements of low, high or unclear risk.
Measures of treatment effect
We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous data in studies where possible, to measure the effects of the intervention and in keeping with the methods of the Cochrane Tobacco Addiction Group. For continuous data we intended to use mean differences (MDs) if outcomes were measured in the same way. We planned to use standardized mean differences (SMDs) to combine trials that measured the same outcomes, but using different methods or scales.
Unit of analysis issues
We used the individual as the unit of analysis in studies. We dealt with unit‐of‐analysis issues using guidance from the Cochrane Handbook (Higgins 2011).
Dealing with missing data
If the proportion of missing data suggested a risk of bias, we reported this. When handling quit rates, we planned to use an intention‐to‐treat analysis where possible, including all participants originally randomized.
Assessment of heterogeneity
We visually explored heterogeneity between effect sizes using tables and forest plots. We planned to pool groups of studies that we considered sufficiently similar, provided that there was no evidence of substantial heterogeneity, as assessed by the I² statistic (greater than 50%) (Higgins 2003). However, we do report two meta analyses with I² results of 72% and 76%, as we deemed the studies sufficiently similar, and could partially account for statistical heterogeneity through further investigation (Higgins 2011).
Data synthesis
We anticipated complexities with data synthesis in this review, similar to those encountered when carrying out the review of legislative smoking bans (Frazer 2016). We have not pooled all studies in a meta‐analysis and instead present a qualitative narrative synthesis of results.
Where we considered studies were sufficiently similar, we report pooled risk ratios, generated using the Mantel‐Haenszel fixed‐effect method, based on quit rates at the longest follow‐up for trials (at least six months from the start of the intervention). We have produced a 'Summary of findings' table to present the smoking prevalence outcome.
Subgroup analysis and investigation of heterogeneity
We considered the following categories for subgroup analyses:
Studies in different specialist settings, for example healthcare facilities, higher education and correctional facilities.
Studies reporting full or partial smoking bans or policies in countries with national anti‐smoking legalisation versus those without national legislation.
Studies extending bans in specialist populations including employees, patients, nursing home residents, students, prisoners and military personnel versus those that do not.
We did not attempt the following prespecified subgroup analyses in this review, as it was not appropriate given the studies identified for inclusion:
Studies which follow the Russell Standard for reporting abstinence outcomes in smoking cessation, including: abstinence, duration, biochemical verification, versus those that do not (West 2005).
Studies that use and do not use biochemically‐validated secondhand smoke outcomes.
Results
Description of studies
See Characteristics of included studies; Table 2; Table 3; Table 4; Characteristics of excluded studies.
1. Characteristics of hospital bans.
| Study ID | Country | Setting | National Ban and Settings ban |
| Alonso‐Colmenero 2010 | Spain | Hospital | National ban: 28/2005. National indoor smoking ban enacted 1st January 2006 banned direct and indirect tobacco publicity and sponsorship; it reduced points of sale, and it banned smoking in enclosed workplaces and public spaces, with exemptions in the restaurant and hospitality sector (Partial ban at time of study). Settings: Hospital policy not described. |
| Etter 2008 | Switzerland, Geneva | Hospital | No national ban. Settings: Smoking prohibited in February 2004 everywhere except smoking rooms. January 2006 smoking rooms removed and smoking totally prohibited inside hospital. |
| Filia 2015 | Australia, Melbourne | Hospital | State ban Victoria: 1st July 2007. All restaurants, cafes, dining areas and shopping centres, enclosed workplaces, covered railway platforms, bus and tram stops and underage music and dance events are smoke‐free. Enclosed licensed premises and outdoor eating and drinking areas (where there is a roof and the wall surface area is more than 75%) must also be smoke‐free as of July 1, 2007. The gambling floors of casinos exempt. Settings: Total smoking ban implemented in the inpatient psychiatric unit in June 2008, including outdoor areas. |
| Fitzpatrick 2012 | Ireland, Dublin |
Hospital | National ban: March 2004. Smoking banned in general workplace, enclosed public places, restaurants, bars, education facilities, healthcare facilities and public transport. However, it is permitted in designated hotel rooms and there is no ban in residential care, prisons and in outdoor areas. Settings: Hospital ban in 2004 following national smoke‐free ban. Total smoke‐free hospital campus policy in 2009. No smoking permitted indoors or outdoors. |
| Gadomski 2010 | USA, New York |
Hospital | National ban: New York State Smoke‐free air act 2002, enacted 2003. Banned smoking in virtually all workplaces and indoor recreational venues. Amendment to the City’s 1995 Smoke‐Free Air Act, the new law banned smoking in all restaurants and most bars regardless of seating and size. The law restricted smoking in some outdoor restaurant and bar seating areas. Settings: Smoke‐free medical campus implemented on July 1, 2006, which included an NRT programme and additional signage. |
| Gazdek 2013 | Croatia, Kopriivnica‐ Krizevci county | Hospital | National ban: November 2008. Smoking officially banned in government buildings, private worksites, educational and healthcare facilities, taxis, and domestic or international air flights after 1999 legislation enacted. Smoking restricted (not banned) on trains, ferries,restaurants, nightclubs and bars, and other public places. 22nd November 2008 law extended to bars, restaurants and cafes. This is not reported in paper. Settings: Smoking bans in healthcare facilities. |
| Harris 2007 | Canada, Ontario | Hospital | National: Not indoor smoke‐free legislation. Ontario's Tobacco Control Act in 1994 banned smoking in all government buildings. Large psychiatric facilities, including MHCP, sought and received special dispensation to allow patients and some staff to smoke in specially ventilated rooms. "Smoking rooms" were already in existence on most wards and some common patient areas at MHCP. The hospital constructed smoking gazebos outside various buildings for patients and staff to use. Ontario smoke‐free indoor legislation implemented in 2006. Settings: Comprehensive tobacco ban. Tobacco products no longer allowed anywhere on 225‐acre grounds after May 6, 2003. |
| Keizer 2009 | Switzerland, Geneva | Hospital | No national ban. Settings: A partial smoking ban established in a psychiatric university hospital, where only 1 ventilated room was made available for smoking for inpatients. Indoor smoking was comprehensively banned for staff January 2002. |
| Martínez 2014 | Spain | Hospital | National ban: 2006 to 2010. Spain had a partial ban on smoking in public places. Offices, schools, hospitals and public transportation were smoke‐free, but restaurants and bars could create a "smokers' section" or allow smoking if they were small (under 100 m²). Extension of ban January 2011 restricted smoking in every indoor public place, including restaurants, bars and cafes. Hotels may designate up to 30% of rooms for smoking; mental hospitals, jails and old people's residences may have public rooms where workers cannot enter. Outdoor smoking is also prohibited at childcare facilities, in children's parks and around schools and hospital grounds. Settings: Smoke‐free centre policy was progressively introduced. Tobacco control programme (2000 ‐ 2012) |
| Morito 2015 | Japan,Fukuoka | Hospital | No national ban. Settings: 2002 to 2006. Introduced smoke‐free zones in hospital. Smoking areas and smoking tables subsequently removed. Hospital became smoke‐free (indoors) in 2007. |
| Ripley‐Moffitt 2010 | USA, North Carolina | Hospital | No national ban. Settings: Tobacco‐free hospital policy introduced 4 July 2007. Employees offered free NRT, signage posted up and no smoking advertising 1 yr. lead in to policy. 100% tobacco‐free campus. |
| Santina 2011 | Spain, Barcelona | Hospital | National ban: National smoking law introduced on January 1st 2006, and indoor smoking banned. Settings ban: not included. Evaluated national ban. |
2. Characteristics of prison bans.
| Study ID | Country | Setting | National Ban and Settings ban |
| Binswanger 2014 | USA | Prisons | National: Enactment varied by state/ordinance. Since 1993 US Supreme Court ruling that suggested exposure of prisoners to environmental tobacco smoke considered "cruel and unusual punishment" in violation of 8th Amendment. Settings: Either smoke‐free (indoor ban), comprehensive (indoor and outdoor), or tobacco‐free policy. |
| Dickert 2015, | USA, New Jersey |
Prisons | National ban: New Jersey’s Smoke‐Free Air Act prohibits smoking in enclosed indoor spaces (2006). March 2010, an amendment banned the use of electronic smoking devices in indoor public places and workplaces and the sale to people 19 years and younger. Settings: NJDOC policy decision for tobacco‐free prisons, including grounds 2012. 13th Feb 2013 policy to ban sales and use of all tobacco products for employees, visitors and prisoners enacted. |
| Etter 2012 | Switzerland | Prison | No national ban. Settings: In prison A, the SHS intervention consisted of an extension of smoke‐free zones and in 2009 smoking allowed everywhere except some indoor workplaces. From 2010 smoking only allowed in cells and outdoors. In prisons B and C in 2009, prisoners were allowed to smoke only in cells, during their outdoor exercise, and in 1 smoking room in prison C. Rules were loosely enforced and respected. There was no policy change regarding SHS in prison B. In prison C, the SHS intervention was limited to better enforcement of the smoking ban in the waiting rooms of the medical service. No cessation programmes in Prison A, inmates charged for NRT, prisons B and C in 2010/2011 medical staff trained to provide smoking cessation counselling and provide NRT. NRT was free in Prison C only. Smoking cessation booklets distributed to all prisons. |
NJDOC: New Jersey Department of Corrections SHS: secondhand smoke
3. Characteristics of hospital bans.
| Study ID | Country | Setting | National Ban and Settings ban |
| Lechner 2012 | USA,Oklahoma | University | No national ban. Settings: 100% tobacco‐free campus policy introduced in July 2008. The use, sale and promotion of tobacco products were prohibited. |
| Seo 2011 | USA, Indiana | University | No national ban. Settings: Indiana university total campus ban began 1 January 2008: smoking prohibited in all indoor and outdoor areas on campus. Smoking prohibited in university vehicles but not prohibited in personal vehicles. Purdue University in West La Fayette allowed smoking at distance of at least 30 ft. from university facilities during study period. |
Results of the search
We searched the literature for this review in June 2015. The database search yielded 1144 records. The Google Scholar search, handsearches, reference lists and information from authors about studies yielded 532 additional records. We excluded 1537 titles and abstracts, and reviewed 139 full‐text papers. Figure 1 provides further information on the identification and screening of relevant records and studies.
1.
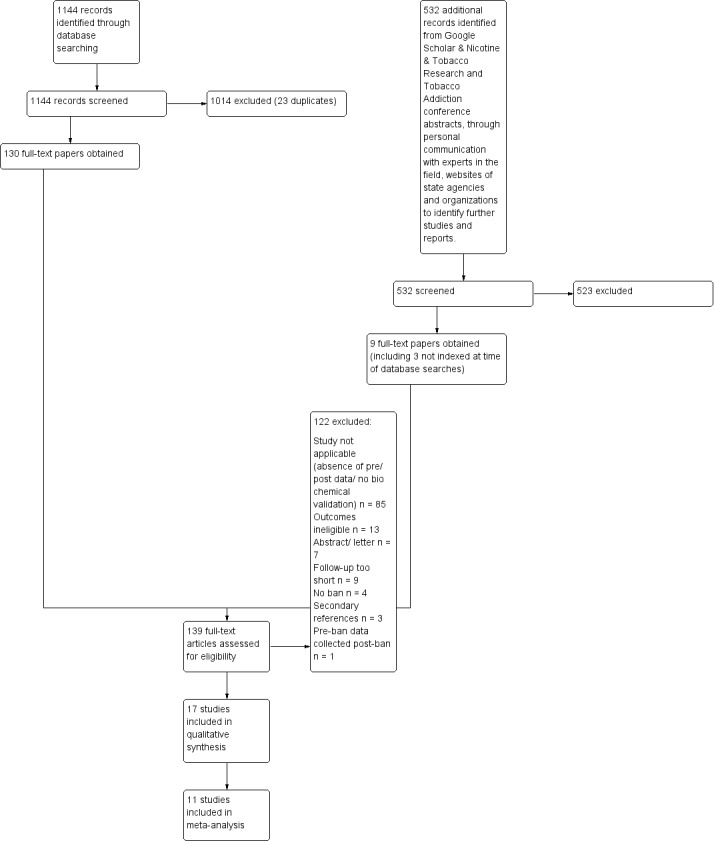
Study flow diagram
Included studies
Seventeen studies met our eligibility criteria. Twelve of these report the impact of smoking policies in healthcare settings (Alonso‐Colmenero 2010; Etter 2008; Filia 2015; Fitzpatrick 2012; Gadomski 2010; Gazdek 2013; Harris 2007; Keizer 2009; Martínez 2014; Morito 2015; Ripley‐Moffitt 2010; Santina 2011). Three studies investigate the effect of smoking policies in prisons (Binswanger 2014; Dickert 2015; Etter 2012); and two studies investigate the effect in university settings (Lechner 2012; Seo 2011).
Eight countries are represented in this review, including the USA (Binswanger 2014; Dickert 2015; Gadomski 2010; Lechner 2012; Ripley‐Moffitt 2010; Seo 2011); Spain (Alonso‐Colmenero 2010; Martínez 2014; Santina 2011); Switzerland (Etter 2008; Etter 2012; Keizer 2009); Australia (Filia 2015); Canada (Harris 2007); Croatia (Gazdek 2013); Ireland (Fitzpatrick 2012) and Japan (Morito 2015).
Eight studies were located in countries or US states that had a national legislative smoke‐free ban and a specialist setting policy or ban (either partial or comprehensive) in place: three in Spain (Alonso‐Colmenero 2010; Martínez 2014; Santina 2011); two in the USA (New York‐ Gadomski 2010; New Jersey‐ Dickert 2015) and one each in Australia (Filia 2015); Ireland (Fitzpatrick 2012); and Croatia (Gazdek 2013) .
Eight studies included in this review were in countries or in US states with no legislative bans, with only a specialist setting tobacco control policy or ban in place, including: three studies in Switzerland (Etter 2008; Etter 2012; Keizer 2009); three in the USA (North Carolina Ripley‐Moffitt 2010; Oklahoma Lechner 2012; Indiana Seo 2011); one in Canada (Harris 2007) and one in Japan (Morito 2015). The final study in the review, Binswanger 2014, included all 50 US States (some with legislative bans and some without) and compared smoking‐related mortality outcomes in prisons with a ban to those without a ban or policy.
The smoking policy interventions included in the specialist settings in this review had to be implemented and evaluated for a minimum period of six months (pre‐intervention data required). The intervention varied from partial indoor tobacco control bans or policies to comprehensive tobacco control bans or policies. In countries with national legislative bans, the local “settings” tobacco control policy or ban sometimes mirrored partial national legislation banning smoking indoors in these specialist settings (Alonso‐Colmenero 2010; Gazdek 2013; Santina 2011). However, a number of studies included in this review evaluated policies in hospitals which implemented more comprehensive smoking bans or extensions of national smoking bans; namely total campus bans and banning indoor and outdoor smoking activities (Filia 2015; Fitzpatrick 2012; Gadomski 2010).
Martínez 2014 evaluated the impact of a number of smoking bans prior to and then following national smoke‐free legislative bans. These hospital smoking bans progressed to a comprehensive indoor and outdoor smoking ban. Binswanger 2014 evaluated the impact of smoke‐free policies in prisons, including smoke‐free policies when indoor smoking was banned, or policies which comprehensively banned smoking both indoors and outdoors, depending on state laws. Similarly Dickert 2015 evaluated the impact of a tobacco‐free policy in a prison banning the sale and use of tobacco products for all employees, visitors and prisoners, again reflecting the New Jersey State ban.
Eight studies with no national legislative smoking bans described varying stages of indoor smoke‐free policies in all of the specialist settings. Harris 2007 evaluated the implementation of a comprehensive smoke‐free ban prohibiting tobacco products from a large maximum security forensic mental health hospital, at a time when psychiatric hospitals were exempted from legislation. Etter 2008 evaluated a policy that provided “designated indoor smoking rooms” progressing to a total prohibition of smoking indoors in a psychiatric hospital. Morito 2015 identified a progressive hospital policy in Japan which introduced smoke‐free zones in a general hospital initially, and then subsequent removal of these zones when the hospital became smoke‐free over a period of five years. Keizer 2009 evaluated a partial smoking ban in a psychiatric unit which permitted patients to smoke in a designated ventilated room; staff smoking was totally prohibited indoors. Ripley‐Moffitt 2010 evaluated a comprehensive tobacco‐free hospital policy which banned smoking indoors and outdoors on a hospital campus.
In a prison setting in Switzerland, Etter 2012 evaluated the effect of increased smoke‐free zones. In one prison, smoking was permitted anywhere with the exception of indoor workplaces initially. The policy was extended one year later to permit prisoners to smoke only in cells and outdoors. This prison was compared to two others with different smoking policies that permitted smoking in cells, during exercise outdoors, and in one of the control prisons smoking was also permitted in a designated smoking room.
Lechner 2012 and Seo 2011 evaluated the introduction of a comprehensive tobacco‐free campus policy in university settings, where the sale, use and promotion of tobacco products were banned. These studies were located in US states with no national legislative smoking bans.
Thirteen studies reported active smoking measures as a primary outcome, including smoking prevalence (smoking rates) and quit rates; four of these studies (Etter 2008; Etter 2012; Keizer 2009; Lechner 2012) also included self‐reported outcomes for environmental tobacco smoke exposures. There were four studies that identified health or mortality as a primary outcome measure (Binswanger 2014; Dickert 2015; Harris 2007; Morito 2015).
We found no randomized controlled studies for inclusion. All included studies are observational in design; three studies use a controlled before‐and‐after design, employing another setting as a comparison (Binswanger 2014; Etter 2012; Seo 2011); 14 studies used uncontrolled before‐and‐after designs (Alonso‐Colmenero 2010; Dickert 2015; Etter 2008; Filia 2015; Fitzpatrick 2012; Gazdek 2013; Keizer 2009; Lechner 2012; Martínez 2014; Morito 2015; Santina 2011; Gadomski 2010; Harris 2007; Ripley‐Moffitt 2010), three of which used a cohort design (Gadomski 2010; Harris 2007; Ripley‐Moffitt 2010). Seo 2011 employed a separate smaller nested cohort study design within the larger controlled before‐and‐after study. Binswanger 2014 and Dickert 2015 used interrupted time series mortality data.
Five studies in this review analysed data on two separate specialist populations (Etter 2008; Etter 2012; Fitzpatrick 2012; Gadomski 2010; Keizer 2009), in their specialist settings of hospitals or prison (i.e. staff and patients or staff and prisoners). The remaining studies report outcomes for one specialist population group: employees, prisoners, inpatients or students.
Further information can be found in the Characteristics of included studies table.
Excluded studies
We excluded from this review studies which did not meet the inclusion criteria. Connell 2010 evaluated the effect of introducing tobacco‐free policies into prisons with smoke‐free policies in Kentucky; however, this study did not include any pre‐ban data. We excluded Pagano 2015 from this review as pre‐ban data were collected after the implementation of the tobacco control policy in some of the healthcare units. We report all reasons for exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
We made explicit judgements of bias according to the criteria in the Cochrane Handbook (Higgins 2011). See Characteristics of included studies table. A summary of the assessments is provided in Figure 2. We consider the study designs used in this review, evaluating a policy‐level health promotion outcome, and the evidence, to be at high risk of bias. However, it must be acknowledged that two of the studies employed mortality data from national registries (Binswanger 2014; Dickert 2015); three studies included a control reference area for comparison (Binswanger 2014; Etter 2012; Seo 2011).
2.
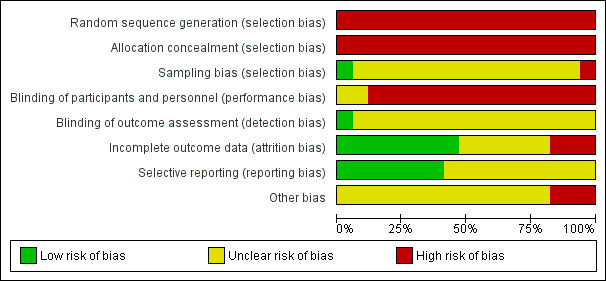
Selection bias
We assessed whether studies used appropriate methods to obtain representative samples of participants. Two studies used nationally representative data from registries (Binswanger 2014; Dickert 2015) and three studies described random sampling methods (Fitzpatrick 2012; Lechner 2012; Santina 2011). Fitzpatrick 2012 employed a quota system to obtain a randomly‐selected sample of staff. Using HR records, they obtained a 10% sample stratified by occupational health grouping. Earlier surveys had also included randomly‐selected methods (Fitzpatrick 2009). A census of inpatients was also achieved in this study and in pre‐ban data collection (Fitzpatrick 2009). Santina 2011 also employed randomization to obtain a sample of staff. If a staff member did not want to participate they were replaced by another, matched for age, sex and occupation. Lechner 2012 reported using a clustered random sampling method, from a list of university courses, to access a sample. Volunteer sampling methods were described by Seo 2011 in the recruitment of students for a longitudinal cohort study, and convenience sampling was employed in larger cross‐sectional surveys at baseline and follow‐up; the sample consisted of white non‐Hispanic students. Ripley‐Moffitt 2010 described a sampling method that involved selecting staff with email addresses; 16 per cent of staff were subsequently reported not to have email addresses.
Blinding
It was not possible to blind participants, as the intervention was a policy or ban and smoking is a visible activity. The use of large national registries of data also negated blinding. Environmental tobacco exposures reported in this review are all self‐reported. Biochemical verification of active smoker status was measured at baseline in two studies (Alonso‐Colmenero 2010; Fitzpatrick 2012). The remaining studies including active smoking measures were all self‐reported. Three studies report using face‐to‐face data collection methods (Alonso‐Colmenero 2010; Fitzpatrick 2012; Santina 2011), with Santina 2011 acknowledging a potential Hawthorne effect bias in using this method for data collection. Etter 2012 indicated that assistance from researchers was available if prisoners were unable to complete questionnaires themselves, but did not report whether this method was employed. Five studies reported using anonymised questionnaires for data collection purposes (Etter 2008; Etter 2012 (staff); Filia 2015; Gazdek 2013; Martínez 2014).
Incomplete outcome data
The use of imputed scores was not reported in any of the studies in this review. Low response rates or high attrition rates were reported over the course of studies in Etter 2012; Gazdek 2013; Martínez 2014; Ripley‐Moffitt 2010; Seo 2011. Retention was encouraged in two cohort studies by offering gift cards to participants (Ripley‐Moffitt 2010; Seo 2011). Fitzpatrick 2009 reported a low response rate for one staff survey in 2002, due to an alternative data collection process.
Selective reporting
Two studies used existing data sets (Binswanger 2014; Dickert 2015); Harris 2007 employed a retrospective chart audit including reported smoking status. Morito 2015 used inpatient admissions details and a chart review to identify inpatient acute myocardial infarction (AMI) cases. Gadomski 2010 accessed staff occupational health records reporting smoking prevalence and used a hospital database of NRT‐prescribing records to identify changes in prescribing patterns for patients. A number of studies identified higher response rates from female staff at either baseline or follow‐up (Martínez 2014; Ripley‐Moffitt 2010; Santina 2011) or a higher response from one occupational health group over another, e.g. nurses (Keizer 2009). Ripley‐Moffitt 2010 reported a 12 per cent smoking prevalence at baseline which was 10 per cent lower than population estimates, suggesting a lower response to the survey among staff who smoked. Fitzpatrick 2009 combined data from two separate patient surveys (1997 and 1998) into one reported data set. Both surveys had been conducted within six months of each other, with no seasonal differences noted.
Two studies verified smoking status at baseline (Alonso‐Colmenero 2010; Fitzpatrick 2012). There is a reliance on self‐reported unverified smoking status in studies included in this review. However, these weaknesses are likely to reflect the methods employed rather than selective reporting by the authors.
Other potential sources of bias
Smoking status variables were self‐reported for the majority of studies reporting active smoke exposure (Etter 2008; Etter 2012; Filia 2015; Gadomski 2010; Gazdek 2013; Keizer 2009; Lechner 2012; Martínez 2014; Ripley‐Moffitt 2010; Santina 2011; Seo 2011). Passive smoke exposure was self‐reported in four studies included in this review (Etter 2008; Etter 2012; Keizer 2009; Lechner 2012). The sample sizes used in a number of studies included in this review are small (Alonso‐Colmenero 2010; Etter 2008; Filia 2015; Harris 2007; Keizer 2009; Morito 2015); however, other studies did employ larger sample sizes (Binswanger 2014; Dickert 2015; Gadomski 2010 (NRT records); Gazdek 2013; Lechner 2012; Seo 2011). Seo 2011 used a matched university for comparison, but acknowledged that smoking prevalence was lower at baseline in the control setting. Lechner 2012 reported that a downward trend in smoking at the universities, after a smoking policy was introduced, could be explained by other activities or secular changes. Martínez 2014 acknowledged that smoking prevalence rates among employees in a cancer centre may be lower than other hospitals, as the participants were an informed group of employees.
Other biases include a change to healthy heart diets in prisons during the period of Dickert 2015. Binswanger 2014, Dickert 2015, Etter 2012 all reported issues with the reallocation of prisoners between prisons during the data collection periods for their studies, to other prisons with more or less stringent or enforced smoking tobacco policies. In addition, higher smoking rates in prisons with poorly‐ventilated areas may have influenced study outcomes.
Etter 2012 reported that due to limited resources, no follow‐up surveys of staff were completed in one of the comparison prisons and that the follow‐up period for the survey of prisoners was only three months. This prison was identified as having difficulties with overcrowding, resulting in nonsmokers being placed in cells with smokers.
Effects of interventions
See: Table 1
Passive smoking
No studies assessed the effect on passive smoke exposures using measurements of cotinine. We identified four observational studies that reported the impact of passive smoke exposure on health and mortality outcomes (Binswanger 2014; Dickert 2015; Harris 2007; Morito 2015). We identified four observational studies providing self‐reported passive smoke exposure in addition to reporting active smoking rates for each specialist setting (Etter 2008; Etter 2012; Keizer 2009; Lechner 2012).
A reduction in passive smoke exposure was reported in all three settings after the introduction of smoking policies or bans restricting or limiting exposure. Lechner 2012 reported reduced smoke exposure at entrances to university campus buildings amongst students, and a greater preference for the smoke‐free environment in Oklahoma. Etter 2008 identified a reduction in duration of SHS exposure per day amongst hospital inpatients in Switzerland, with staff also reporting significant reductions in SHS exposure. In this study nonsmokers reported reduced SHS exposure after the introduction of a partial smoking ban, with no further decrease in exposure soon after a total indoor smoke‐free ban was introduced and 27 months after the partial ban was introduced. Similar results were reported by Keizer 2009, who reported that staff who smoked were less bothered about SHS exposure than nonsmokers in this Swiss study. In the prison setting in Switzerland, Etter 2012 observed comparable results, with reduced exposure time among prisoners and staff after the introduction of a smoking policy and restricted smoking. No significant reductions in reported SHS exposure were identified by prisoners in the two comparison prisons in this study. Staff did acknowledge reduced smoke exposure in the control prison sites with fewer restrictions ( Analysis 1.1).
1.1. Analysis.
Comparison 1 Passive exposure (narrative), Outcome 1 Passive smoke exposure.
| Passive smoke exposure | |||||
|---|---|---|---|---|---|
| Study | Country | Setting | National Ban and Settings ban | Participants | Results |
| Etter 2008 | Switzerland, Geneva | Hospital | No national ban. Settings: Smoking prohibited in February 2004 everywhere except smoking rooms. January 2006 smoking rooms removed and smoking totally prohibited inside hospital. |
Patients 2003 Pre: n = 49 2004 Post: n = 54 2005 Post: n = 66 2006 Post: n = 77 Staff Pre: n = 57 2004 Post: n = 54 2005 Post: n = 53 2006 Post: n = 57 |
Among nonsmokers, ETS reduced in bedrooms after partial ban, but did not decrease after total ban. After total ban, self‐reported exposure to ETS decreased from 69 min/day (2005) to 12 min/day (2006) after total ban, P = 0.012. 52.8% of respondents agreed with smoking restrictions post‐ban in 2006. Patients reported statistically significant difference in opinion pre‐/post‐ban "tobacco smoke is source of conflict with staff", 24.7% (pre), 36.4% post, P = 0.005. Fewer patients reported cohabitation between smokers and nonsmokers is very difficult post‐ban 54.4% (pre), 44.9% (post), P = 0.033. Patients locked in rooms identified prohibition on smoking "hard to bear" 75% (pre) and 78% post. After total ban number of patients getting angry with staff because of policy increased 4.5% (pre) 24.5% (post), P = 0.02, OR 6.8, 95% CI 1.2 to 47.3. No significant increase in staff reporting that patients were angry. 32.7% of staff in 2005 and 42.8% of staff in 2006 (P = 0.28) agreed with the statement that "after totally prohibiting smoking in clinic they would face strong protest from patients". Question not asked after total ban. After total ban staff reported that patients still smoked in bedroom (80.7%) and left clinic to buy cigarettes (82.4%). |
| Etter 2012 | Switzerland | Prison | No national ban. Settings: In prison A, the SHS intervention consisted of an extension of smoke‐free zones and in 2009 smoking was allowed everywhere except some indoor workplaces. From 2010 smoking only allowed in cells and outdoors. In prisons B and C in 2009, prisoners were allowed to smoke only in cells, during their outdoor exercise and in 1 smoking room in prison C. Rules were loosely enforced and respected. There was no policy change regarding SHS in prison B. In prison C, the SHS intervention was limited to better enforcement of the smoking ban in the waiting rooms of the medical service. No cessation programmes in Prison A, inmates charged for NRT, prison B and C in 2010/2011 medical staff trained to provide smoking cessation counselling and provide NRT. In Prison C NRT was free in this prison only. Smoking cessation booklets distributed to all prisons. |
Prisoners Prison A Pre: n = 70 Post: n = 60 Prison B Pre: n = 27 Post: n = 30 Prison C Pre: n = 116 Post: n = 66 Staff Prison A Pre: n = 51 Post: n = 48 Prison B Pre: n = 27 Post: n = 24 Prison C Pre: n = 126 Post: n = 0 |
In prison A, prisoners and staff reported less exposure to SHS in 2011 than in 2009: 31% of prisoners were exposed to smoke at indoor workplaces in 2009 vs 8% in 2011 (P = 0.001); in common rooms: 43% vs 8%, (P < 0.001); but not outdoor workplaces. No changes were observed in prisons B and C. All prisons, staff reported reductions in SHS exposure. Prison A: median significant decrease in time of smoke exposure 25 mins/day (2009) reduced to 2 mins (2011), P < 0.001. No significant difference when compared to prison B. Prisoner ETS exposure significantly reduced in follow‐up in prison A in cafeteria, common rooms, break rooms and indoor workplaces, but not outdoor workplaces. |
| Keizer 2009 | Switzerland, Geneva | Hospital | No national ban. Settings: A partial smoking ban established in a psychiatric university hospital, where only 1 ventilated room was made available for smoking for inpatients. Indoor smoking was comprehensively banned for staff January 2002. |
Staff Pre‐ban: n = 110/281 Post‐ban: n = 85/160 Patients Pre‐ban 2001: n = 91/167 Post‐ban 2005: n = 134/263 |
There was a perceived decrease in the amount of smoke in the hospital reported by staff (and patients), P = 0.00005. Smokers less bothered by SHS exposure than nonsmokers, P = 0.005 amongst staff. |
| Lechner 2012 | USA, Oklahoma | University | No national ban. Settings:100% tobacco‐free campus policy introduced in July 2008. The use, sales and promotion of tobacco products was prohibited. |
Students Pre: n = 1185 2008 n = 1197 2009 n = 1257 2010 n = 1242 |
Results indicated that exposure to smoke at an entrance to a campus building had significantly decreased over the 4‐year assessment period, F (3, 4908) = 126.38, P < 0.001, η2 = 0.071. Students reported significant increase in preference to socialise in smoke‐free environment F (3, 4836) = 4.48, P = 0.004, η2 = 0.002. Noted in 2008, and 2010 but not in 2009. Significant agreement over time that campus be smoke‐free, P < 0.001. |
Health and smoking‐related mortality outcomes
Four studies in this review evaluated the impact of smoking policies on health and smoking‐related mortality outcome measures in prisons and hospitals (Binswanger 2014 prison; Dickert 2015 prison; Harris 2007 secure mental hospital; Morito 2015 hospital) ( Analysis 2.1 ). Binswanger 2014, in a review of mortality data, reports that mortality associated with smoking‐related illness was reduced in prisons which had a smoking ban established for a period of nine or more years, when compared to prisons with no smoking policies. They identified 48 states in the USA with a smoking ban and prison policies in place in 2011, an increase from 25 states in 2001 (baseline).
2.1. Analysis.
Comparison 2 Health and mortality outcomes (narrative), Outcome 1 Health and mortality outcomes.
| Health and mortality outcomes | |||||
|---|---|---|---|---|---|
| Study | Country | Setting | National Ban and Settings ban | Participants | Results |
| Binswanger 2014 | USA | Prisons | National: Enactment varied by state ordinance. Since 1993 US Supreme Court ruling that suggested exposure of prisoners to environmental tobacco smoke considered "cruel and unusual punishment" in violation of 8th Amendment. Settings: Either smoke‐free (indoor ban), comprehensive (indoor and outdoor) or tobacco‐free policy. |
Prisoners n = 287 prisons n = 14,499 prisoners |
Smoking bans in place for 9 or more years were associated with reductions in smoking‐related mortality: RR 0.89, 95% CI 0.85 to 0.94. Cancer deaths: RR 81, 95% CI 0.74 to 0.90. Pulmonary deaths RR 0.66, 95% CI 0.54 to 0.80 compared to states with no bans. After adjusting for deaths from smoking‐related causes in the population, little change in point estimates, 95% CI were marginally wider. No significant results when analysed deaths from other causes 2001 to 2011, RR 1.05, 95% CI 1.00 to 1.09. 2004, 75.8% had ever smoked. Current male smokers aged 35 ‐ 64 years = 38.5%, and 17.7% for 65 years and older. Current female smokers: 46.7% (35 ‐ 64 years) and 5.9% (65 years and older). In 2001 25 states had a smoking ban. By 2011 48 states had a smoking ban. 44 banned smoking indoors and 39 banned smoking or tobacco outdoors. |
| Dickert 2015 | USA, New Jersey | Prisons | National ban: New Jersey’s Smoke‐Free Air Act prohibits smoking in enclosed indoor spaces (2006). March 2010, an amendment banned the use of electronic smoking devices in indoor public places and workplaces and the sale to people 19 years and younger. Settings: NJDOC policy decision for tobacco‐free prisons, including grounds 2012. 13th Feb 2013 policy enacted to ban sales and use of all tobacco products for employees, visitors and prisoners. |
Prisoners n = 13 prisons Census prisoners Jan ‐ June 2005 n = 26,239, prisoners special needs n = 3533 Census Jan ‐ June 2011, n = 22,318, prisoners special needs n = 3020 |
Total mortality was 3 times higher for persons with special health needs compared to all prisoners. Annual mortality rate decreased 13% from 232 to 203/100,000 population between 2005 and 2013 after smoking ban introduced. The mortality rate for persons with special mental health needs decreased 48% from average of 676/100,000 to 353/100,000 in 18 months after ban introduced. |
| Harris 2007 | Canada, Ontario | Hospital | National: Ontario's Tobacco Control Act in 1994 banned smoking in all government buildings. Large psychiatric facilities, including MHCP, sought and received special dispensation to allow patients and some staff to smoke in specially ventilated rooms. "Smoking rooms" were already in existence on most wards and some common patient areas at MHCP. The hospital constructed smoking gazebos outside various buildings for patients and staff to use. Ontario's national smoke‐free legislation adopted in 2006. Settings: Comprehensive tobacco ban. Tobacco products no longer allowed anywhere on 225‐acre grounds after May 6, 2003. |
Patients n = 119 n = 83 maximum security division n = 32 open wards |
89% male, mean age 46.8 years (SD 11.1 yrs). Among 23 smokers rated as having signs of compromised cardiopulmonary health at their annual medical check‐ups in the year before the tobacco ban, 17 received a clear/healthy assessment at their annual physical examinations in the year after (P < 0.05, Fisher's exact test). For the majority of patients who were in the maximum security forensic division, the tobacco ban was associated with almost no detectable ill effects with some clear benefits. The ban was associated with an increase in physical aggression towards staff members in open wards only F (1,106) = 4.33, P < 0.05. Clozapine prescribing increased in smokers and weight increased in max security patients. |
| Morito 2015 | Japan, Fukuoka | Hospital | No national ban. Settings: 1981 ‐ 2002 hospital provided separate facilities for smokers and nonsmokers. 2003 to 2006 introduced smoke‐free zones in hospital. Smoking areas and smoking tables subsequently removed. Hospital became smoke‐free (indoors) in 2007. |
Patients Pre‐ changes 2002: n = 4 Stage 1 2003 ‐ 2006: n = 14 Stage 2 2007 ‐ 2010: n = 4 Stage 3 2011 ‐ 2014: n = 3 |
AMI data from January 2002 ‐ June 2014. Patients with an in‐hospital onset of AMI were defined as those who had AMI but were not under the care of Departments of Cardiology or Emergency. N = 25 patients identified in total Pre changes 2002: n = 4 Stage 1 2003 ‐ 2006: n = 14 Stage 2 2007 ‐ 2010: n = 4 Stage 3 2011 ‐ 2014: n = 3 P for trend = 0.010. n = 6 died ( Age 76 (SD 7)) ( 3 were smokers) n = 19 survived (Age 68 (SD 9)) (12/19 smokers) 10/ 25 AMI after surgical operation. 16 men and 9 women. No statistically significant difference in patient characteristics between operation and non‐operation groups except for DL (lipid‐lowering therapy). No differences between smoking and nonsmoking groups except for DL. Increasing nonsmoking policy decreased in hospital onset of AMI. |
Dickert 2015 detected significantly higher smoking rates amongst prisoners with mental health needs in their review of New Jersey mortality data. Significant annual reductions in smoking‐related mortality in prisons were identified for all prisoners, and particularly for those with a diagnosed mental illness, after the introduction of smoking bans. However Dickert 2015 acknowledges that the changes may be confounded by other factors in prisons, including improved healthy heart diets introduced between 2005 and 2007, during the period of the study.
Within the hospital setting, Morito 2015 identified a significant reduction in the onset of AMI inpatient events after the introduction of a phased smoking policy over a 12‐year period; four cases detected in 2002 prior to any policy, 14 cases detected between 2003 to 2006 and seven cases occurring during a seven‐year period after the hospital became smoke‐free. The reduction was significant after statistically adjusting for smoking status and other confounders, with the exception of dyslipidaemia.
Harris 2007's retrospective audit of 119 inpatients' charts in a secure mental hospital in Canada reported improved health assessments one year after the introduction of a hospital campus‐wide smoking ban for 17 of the 23 smokers. No smoking prevalence data were reported in the study, but smoking status was identified from chart information in this study. The identified health effects included improved cardiopulmonary health assessments for 17 of the 23 inpatients.
Active smoking rates
The effect of smoking policies in specialist settings on smoking prevalence amongst some participant groups are reported in 13 studies in this review. Ten studies are based in hospital settings (Alonso‐Colmenero 2010; Etter 2008; Filia 2015; Fitzpatrick 2012; Gadomski 2010; Gazdek 2013; Keizer 2009; Martínez 2014; Ripley‐Moffitt 2010; Santina 2011), one study reported smoking rates in a Swiss prison (Etter 2012), and Lechner 2012 and Seo 2011 reported smoking rates in university settings in two US states ( Analysis 3.1).
3.1. Analysis.
Comparison 3 Active smoking (narrative), Outcome 1 Active smoking rates.
| Active smoking rates | |||||
|---|---|---|---|---|---|
| Study | Country and Setting | Population | National Ban | Outcomes | B io chemical verification |
| Alonso‐Colmenero 2010 | Spain | Hospital | Yes | N = 135 smokers in study. No significant difference reported at baseline. n = 53 smokers identified as smoking in hospital in study. 2005: 34.2% of the 53 smoked in hospital (95% CI 22.6 to 45.8). 2006: 45.1% of the 53 smoked in hospital after the policy (95% CI 31.9 to 58.3), P = 0.26. |
Cotinine measure define smoker |
| Alonso‐Colmenero 2010 | |||||
| Etter 2008 | Switzerland,Geneva, Psychiatric hospital |
Staff | No | Pre‐ban n = 57 staff Post‐ban 2004: n = 54/55, 2005: n = 53/63, 2006: n = 57/62. Participation rates 84.1% to 100%. Current prevalence of smokers unchanged over time. 26.3% (baseline) and at final follow‐up. Significantly more staff perceived rules about smoking were too strict. This changed over time as the smoking ban increased, 7.0% at baseline to 59.6% (final follow‐up), P < 0.001. Rules on smoking not respected (staff and patients) 51.36% at baseline and 16.1% (partial ban 2005) and 32.6% ( total ban 2006), P < 0.001. |
None |
| Etter 2008 | Patients | 73.5% of patients were daily smokers 2003, reduced to 65.8% in 2006. No significant change in mean number of cigarettes 2003 and post‐ban 2006. 24.1 vs 23.7, P = 0.81. Increased quit attempts reported 2.2% in 2005 to 18.4% in 2006, P = 0.01, OR 10.1, 95% CI 1.21 to 222.7 (wider interval). |
|||
| Etter 2012 | Switzerland, Prisons |
Staff | No | Response rates among staff higher than prisoners. Ranged from 40% to 77% over time in the 3 prisons. Majority of staff surveyed were men. No follow‐up in Prison C for staff. In prison A, staff smoking reduced from 10% to 6% at follow‐up. In prison B, staff smoking increased from 26% to 38% at follow‐up. |
None |
| Etter 2012 | Prisoners | Response rate 17% to 44% over period. Prisoner smoking unchanged. At baseline prison A 75% smoked (n = 52/70) and 72% (n = 43/60) at follow‐up. Prison B 69% (19/27) smoked 2009 and 57% (17/30) in 2011. Prison C 58% (67/116) at baseline and 56% 40/66) 2011. No significant change detected in any of the prisoners in smoking status, quit attempts or relapse. Smoking behaviour prison A: more prisoners reported receiving medical help to quit smoking in 2011 (20%) than in 2009 (4%, P = 0.012). Prison A compared to Prison B, prisoners felt that staff should do more to help quit attempts, P = 0.015. In prison A, prisoners and staff reported less exposure to SHS in 2011 than in 2009: 31% of prisoners were exposed to smoke at workplaces in 2009 vs 8% in 2011 (P = 0.001); in common rooms: 43% vs 8%, (P < 0.001). No changes were observed in prisons B and C. |
None | ||
| Filia 2015 | Australia, Melbourne | Hospital | Yes | Before the totally smoke‐free policy, 69.6% smoked, with 67.7% smoking more when admitted to the psychiatry ward smoking average 18.1 cigs/day. (Alternatives to smoking identified included use of NRT, having a designated smoking area, keeping busy). After the totally smoke‐free policy, 57.7% smoked heavily before hospital (mean cigarettes/day = 24.9), with consumption reduced after admission to a totally smoke‐free psychiatric unit (mean cigarettes/day = 8.3). 5.8% of patients reported quitting since admission following the ban. |
None |
| Filia 2015 | |||||
| Fitzpatrick 2012 | Ireland, Dublin | Hospital Staff |
Yes | Pre‐ban data: smoking prevalence rates in staff : 1998: 27.4%; 2001: 17.3%; 17.8% of staff reported smoking in 2006 (post‐1st ban and pre‐2nd phase) and this significantly reduced to 10.7% in 2010, P = 0.02. Significantly in female staff 17.6% vs 9.5%, P = 0.02 and in age group 30 ‐ 39 years. Positive attitude among staff (52.4% vs 83.3%, P < 0.001) to the campus‐wide ban increased significantly between 2006 and 2010; the greatest increase was seen in doctors. Campus ban resulted in a positive attitude amongst staff irrespective of smoking status. When perception of own role in implementation was examined, younger staff were less likely to agree they had a role, while ex‐smokers were more likely to agree they had a role in implementation. Nurses more likely to agree than all other occupational groups. |
None |
| Fitzpatrick 2012 | Patients | Pre ban data: smoking prevalence in patients: 1997/1998: 24.2%; 2002: 15.5%; 2004: 24.5%. No significant change in patients smoking at follow‐up in 2010 after total campus ban introduced in 2009: 22.7% vs 18.0% (2006), P = 0.22. Reducing trends noted for men and women, but not statistically significant. Positive attitude of patients (58.6% vs 84.2%, P < 0.001) to the campus‐wide ban increased significantly between 2006 and 2010. Univariate analysis of factors associated with agreement with campus ban significantly associated with being a non‐ or ex‐smoker (patients), but not current smokers, P = 0.286. Multivariate analysis identified being aged 60 years or older and being a current smoker as significant. |
Patients with CO levels > 10 ppm were considered to be current smokers. | ||
| Gadomski 2010 | USA, New York Hospital |
Staff | Yes | Cohort of 489 hospital employees 2005 and 2007, 12% reported smoking in 2005 and 7.5% in 2007 (McNemar was significant P < 0.001). 2006 not reported. Including all hospital employees reporting any 1 year during their anniversary dates, the self‐reported smoking rates were 14.3% (n = 624) in March ‐ June 2005, 14.8% (n = 661) in March ‐ June 2006, and 9.4% (n = 1112) in March ‐ June 2007 (P < 0.0002). | None |
| Gadomski 2010 | Patients (NRT use) | No change in % patients signing out against advice. 69.8% inpatients received brief intervention post‐ban. NRT orders tripled post‐ban. Inpatient orders increased 832 in 2 years pre‐ban to 2475 in 2 years post‐ban. The Chow test is highly significant for break point in June 2006, P = 0.008. 1 month prior to ban. | |||
| Gazdek 2013 | Croatia, Kopriivnica‐ Krizevci county Hospital |
Staff | Yes | Baseline smoking prevalence 34.3% reduced to 26.4% 2011. A reduction of 7.9%. Reduction in population 1994 to 2005 was 5.2%. Larger change in non‐health workers 39.2% to 26.4% (Change 12.8%). Number of cigarettes decreased per person from 15 to 12 per day. Percent of < 10 cigs consumed/day increased 33.7% to 57.4% in first 2 years of Act. Decrease greatest 2 ‐ 6 years after ban. |
None |
| Gazdek 2013 | |||||
| Keizer 2009 | Switzerland, Geneva Psychiatric hospital |
Staff | No | No significant change in staff smoking prevalence 2001 and 2005. 2001 30.8% vs 29.9% 2005, P = 0.94. Daily consumption of cigarettes among staff: 13% of staff were heavy smokers (> 20 /day) compared to 53.5% of patients, P < 0.001. |
None |
| Keizer 2009 | Patients | No significant changes in current smoking among patients post‐ban (n = 86) 72.1% vs 65.2% (n = 62), P = 0.54. Daily consumption of cigs by patients was 29.47 (SD 16.79) and 17.83 (SD 13.26) for staff, P < 0.001. 13% of staff were heavy smokers (> 20/day) compared to 53.5% of patients, P < 0.001. 34.9% of patients and 52.2% of staff were moderate smokers. Patients displayed an increased desire to stop smoking post‐ban . Trends in patient smoking showed initial decrease in consumption but returned by day 10. Inconclusive as may be due to heavy‐smoker cohort. Increased smoking post‐ban (qualitative) identified boredom, waiting and mental state as reasons. Decreased smoking was explained by restrictions (smoking rooms), lack of cigarettes, tiredness, treatment, decrease in tension, less desire to smoke and respect for others. |
None | ||
| Lechner 2012 | USA, Oklahoma |
University | No | Significant reduction in percentage of more frequent smokers over time Chi² = 8.53 (3, n = 4947), P = 0.036; especially between years 2009 and 2010, Chi² =7.06 (1, n = 2486), P = 0.009, and between 2007 and 2010: Chi² = 5.00 (1, n = 2454), P = 0.025. Proportion of smokers reduced but NS. Significant decreases in the proportion of more frequent smokers occurred in men, Chi² = 14.58 (3, n = 2290), P = 0.002, but not women. Significant decrease in the proportion of less frequent smokers across assessment points, Chi² = 20.87 (4, n = 4947), P < 0.001. Significant decrease occurred between years 2007 and 2010, Chi² = 15.38 (1, n = 2454), P < 0.001. Results indicated that exposure to smoke at an entrance to a campus building had significantly decreased over the 4‐year assessment period, F (3, 4908) = 126.38, P < 0.001, η2 = 0.071. Students reported significant increase in preference to socialise in smoke‐free environment F (34836) = 4.48, P = 0.004, η2 = 0.002. Noted in 2008 and 2010, but not in 2009. Significant agreement over time that campus be smoke‐free, P < 0.001. |
None |
| Lechner 2012 | |||||
| Martínez 2014 | Spain | Hospital | Yes | Smoking prevalence decreased from 33.1% (95% CI 29.3 to 36.9) to 30.5% (95% CI 26.3 to 34.7) and in 2012 22.2% (95% CI 16.7 to 27.6), P < 0.005. Prevalence decreased in all hospital groups. Decreased amongst women 35.1% Baseline to 33.0% (1st ban), 23.1% (2nd ban), P = 0.009, and in aged > 35 years 31.9% baseline, 23.3% (1st ban), 16.3% (2nd ban), P = 0.0001. Smoking decreased in men, but not statistically significant. Smoking reduced in all staff groups, not statistically significant. Smoking patterns: occasional smokers increased 2‐fold. 12.1% to 24.5% (2nd ban), P = 0.012. No clear trend in number of cigs or time to first cig reported. First cig after awakening ≤ 30 mins 3.6% at baseline and 39.1% (2nd law), P < 0.001. Readiness to quit 60.3% baseline, 28.2% (1st ban), 11.5% (2nd ban), P < 0.001. Significant reduction in concern about tobacco use, readiness to fix date to quit and, consulted professional to quit and refrain from smoking in working hrs post‐bans. Attitude to ban: agreed with policy P < 0.001, and parents should set example. Support for the tobacco control policies increased from 59% at baseline to 80.5% following the passage of the 2nd bill. |
None |
| Martínez 2014 | |||||
| Ripley‐Moffitt 2010 | USA,
North Carolina Hospital |
Staff | No | Total sample was 5534, with 2024 respondents to initial survey, of which 307 were current smokers or had quit in preceding 6 months. Follow up n = 210 smokers agreed to be interviewed at 6 months and 1 year post‐ban. n = 166 responded at 6 months. Of 179 participants in study who were smokers, 45% reported quit attempt in previous 6 months. At 6 months, of the 133 participants currently smoking, 53% reported quit attempt. At 1 yr, 39 participants reported not smoking (18.5%). Of the 117 participants who were current smokers at 12 months, 48% reported attempts to quit during preceding 6 months. |
None |
| Ripley‐Moffitt 2010 | |||||
| Santina 2011 | Spain, Barcelona | Hospital | Yes | The number of workers smoking decreased from 35.2% to 27.4%, P < 0.05. This reduction was seen across all hospital workers, less in nursing staff. People only smoked in smoking areas, P < 0.0001. Policy supported by smokers and nonsmokers. 8.2% received help to quit pre‐ban, 19.7% post‐ban, P = 0.02. |
None |
| Santina 2011 | |||||
| Seo 2011 | USA,
Indiana University |
Students | No | Prevalence and tobacco consumption fell in Indiana (pre‐ban: 16.5%; post‐ban: 12.8%) and increased at Purdue (control) during the same time period. In addition, perceptions of peer tobacco use and smoking norms improved at Indiana University. Peer tobacco use: significant decrease in percentage of Indiana students who perceived 26% of students or more were smoking, P < 0.001. Control: significant increase in perceived smoking, P ≤ 0.001. Percentage of friends smoking decreased in Indiana, P < 0.001. Longitudinal panel comparisons samples: n = 170 for Indiana and n = 128 for Purdue. Significant declines in number of cigs smoked in Indiana post‐policy, ‐5.0, P < 0.05, compared to Purdue. Indiana students had significant increases in agreement that smoking regulation is good, P < 0.05; should be banned on all university property, P < 0.05, compared to Purdue for both fixed‐effect and random‐effects modelling. |
None |
| Seo 2011 | |||||
Five of these studies included outcomes for two populations: employees and patients in hospitals (Etter 2008; Fitzpatrick 2012; Gadomski 2010; Keizer 2009) and employees and prisoners in prison (Etter 2012). Inconsistent evidence emerged within hospital and prison settings.
All studies reporting active smoking rates used uncontrolled before‐and‐after study designs, with the exception of Etter 2012 and Seo 2011, which had comparison groups.
Hospital settings
Keizer 2009 did not detect a significant reduction in smoking rates post‐ban in a psychiatric hospital in Switzerland. The study did identify that consumption of cigarettes amongst staff reduced post‐ban, but no difference was reported in patient smoking rates. Trends in patient smoking rates reported an initial decrease in consumption, but a return to usual levels by day 10 of admission. Respondent's qualitative responses identified the following reasons for increased smoking rates: boredom, mental health issues including stress, cravings, or simply due to being in hospital.
Etter 2008 did not detect a reduction in smoking rates for either staff or patients after either a partial or a subsequent total smoking policy was introduced in a psychiatric hospital in Switzerland. An increase in quit attempts by patients was reported. However, the reported confidence interval is very wide and the sample size in the study was small.
Whilst Fitzpatrick 2012 detected a reduction in patient smoking rates, there was no significant difference after the introduction of an additional smoke‐free campus ban. A significant reduction in staff smoking rates was reported, especially amongst female staff and those aged 30 to 39 years after the further campus ban.
A significant reduction in smoking rates amongst staff was reported in Gadomski 2010's cohort study after the introduction of a hospital policy in New York. Whilst no baseline smoking prevalence data were available for patients before the policy, NRT prescribing patterns for patients tripled after the introduction of the policy and no increase in patients leaving the hospital against advice was observed. Gazdek 2013 reported significant reductions in staff smoking rates and tobacco consumption, with the highest decrease in the period two to six years after the hospital policy was introduced in 1999. The national ban was introduced in 2008 during the period of the study. Martínez 2014 also detected significant reductions in staff smoking rates, similar to Fitzpatrick 2012; reductions in 2010 were greater amongst female employees and in those aged over 35 years compared to 2006. Smoking rates decreased in men, but not significantly.
Similar reductions in staff smoking rates were reported by Santina 2011 for all staff groups, with the exception of nursing staff. Significant increases in quit attempts and readiness to quit were reported by Martínez 2014, whilst Ripley‐Moffitt 2010 observed increased quit attempts in their cohort over time. Santina 2011 detected significant increases in the provision of help and assistance with quitting smoking.
Alonso‐Colmenero 2010 did not observe any statistically significant reduction in the number of patients who smoked during their inpatient stay after a policy was introduced. All patients in the study population were smokers and the authors identified 55 patients who smoked whilst in hospital at some point during the study. The percentage of these 55 patients smoking during their hospital stay increased after the hospital policy was introduced.
Prison settings
One study reported smoking prevalence rates in prison. Etter 2012 identified little change in smoking prevalence among staff or prisoners, with no significant change in quit rates either in the intervention prison or when compared to the two control prisons. Staff smoking rates increased in one comparison prison during the reporting period of the study. Prisoners in the intervention prison reported receiving more medical help to quit smoking after the introduction of the smoking policy. NRT in this prison was not free, unlike one of the comparison prisons.
University settings
Finally, within the specialist university setting some positive impacts of campus bans were observed. Lechner 2012 identified a significant reduction in “more frequent smokers” who were male, following the introduction of a campus ban at Oklahoma university; however, this was not observed in women. "More frequent smokers" were defined in this study as individuals who had smoked over 100 cigarettes in a lifetime and who had consumed cigarettes on at least 10 of the last 30 days.
Similarly, Seo 2011 detected a significant reduction in smoking rates at an Indiana university after the introduction of a campus policy, whereas smoking rates increased at Purdue University (La Fayette), which acted as the control. However, it should be noted that the baseline levels of smoking were different across universities. Students also reported significant reductions in peer smoking in Indiana compared to Purdue University. The longitudinal cohort component of this study identified a significant decline in the number of cigarettes consumed in Indiana when compared to Purdue.
Effect on active smoking rates
Due to heterogeneity, it was not possible to conduct a meta‐analysis and pool data from all of the 17 observational studies included in this review. We assessed the impact of smoking policies using uncontrolled before‐and‐after data from 11 of the studies in this review. Eight studies assessed the effect of smoking bans on smoking prevalence rates in hospital settings (Alonso‐Colmenero 2010; Etter 2008; Filia 2015; Fitzpatrick 2012; Gazdek 2013; Keizer 2009; Martínez 2014; Santina 2011). One of the studies assessed smoking prevalence in the prison setting (Etter 2012); only before‐and‐after data from the intervention prison are included in this analysis. The final two studies assessed smoking prevalence in university settings (Lechner 2012; Seo 2011). Only before‐and‐after data from Seo 2011 are used in analyses, with data from the control university and from the smaller nested cohort study excluded. The implementation of policies may have varied from study to study, but all measured the effect of the settings‐based policy on active smoking rates.
Four studies assessed outcomes for patient groups (Alonso‐Colmenero 2010; Etter 2008; Filia 2015; Keizer 2009), and three for hospital employees (Gazdek 2013; Martínez 2014; Santina 2011). Fitzpatrick 2012 assessed outcomes for both staff and patient groups, and therefore the data from this study have been split into these two groups and entered into meta‐analyses separately. Included pre‐ban data for the analyses of staff and patients is reported in Fitzpatrick 2009, prior to the first hospital ban in 2004.
There was evidence of substantial heterogeneity when pooling all 11 studies (I² = 72%), so we report estimates by setting (hospital/university/prison).
We found an effect of a smoking policy on reducing smoking rates across eight hospital‐based studies (Alonso‐Colmenero 2010; Etter 2008; Filia 2015; Fitzpatrick 2012Gazdek 2013; Keizer 2009; Martínez 2014; Santina 2011), with a risk ratio (RR) of 0.75, (95% confidence interval (CI) 0.69 to 0.81; n = 5986; I² = 68%) (Figure 3; Analysis 4.1). Heterogeneity in this subgroup was attributable to the large reduction in smoking amongst hospital staff in Fitzpatrick 2012. The baseline survey used in the analysis preceded the introduction of a comprehensive national ban and hospital ban by over five years, and may reflect secular change in smoking. Similarly in Gazdek 2013, baseline pre‐ban data were collected during this period; however the hospital policy was introduced the following year after the introduction of a partial legislative smoking ban, progressing to a comprehensive smoke‐free ban nine years later during the period of the study. There was also evidence of reduced smoking in the university setting (Lechner 2012; Seo 2011), RR 0.72, 95% CI 0.64 to 0.80; n = 6369, I² = 59%. Only one small study was included in the prison subgroup (Etter 2012), which in contrast showed no evidence of change (RR 0.99, 95% CI 0.84 to 1.16; n = 130).
3.
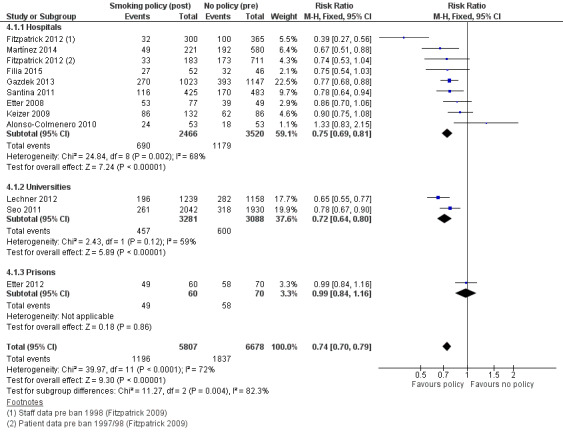
Comparison of active smoking rates in hospitals, universities and prison settings
4.1. Analysis.
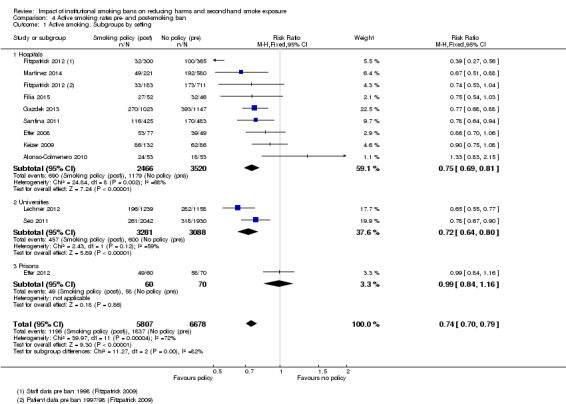
Comparison 4 Active smoking rates pre‐ and post‐smoking ban, Outcome 1 Active smoking. Subgroups by setting.
Subgroup analysis; staff and patient smoking rates in hospitals
The effect of smoking policies in hospital settings was compared between two participant groups (staff and patients). Four studies assessed the impact on staff smoking rates (Fitzpatrick 2012; Gazdek 2013; Martínez 2014; Santina 2011) and five studies assessed the impact on patient smoking rates (Alonso‐Colmenero 2010; Etter 2008; Filia 2015; Fitzpatrick 2012; Keizer 2009). The Alonso‐Colmenero 2010 study measured the effect of a policy on the number of smokers who actively smoked as inpatients in hospital. .
There was evidence of a pooled effect of policies on reducing active smoking rates in both staff (RR 0.71, 95% CI 0.64 to 0.78; n = 4544; I² = 76%) and patients (RR 0.86, 95% CI 0.76 to 0.98; n = 1442; I² = 20%) (Analysis 4.2). Heterogeneity remained in the staff subgroup, attributable to Fitzpatrick 2012 as noted above.
4.2. Analysis.
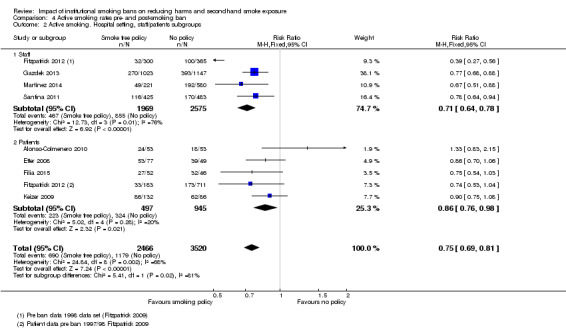
Comparison 4 Active smoking rates pre‐ and post‐smoking ban, Outcome 2 Active smoking. Hospital setting, staff/patients subgroups.
Subgroup analysis; status of national legislation
Six studies assessed the impact of smoking policies on active smoking rates in countries where a national smoking ban existed during the period of data collection (Alonso‐Colmenero 2010 Spain; Filia 2015 Australia; Fitzpatrick 2012 Ireland; Gazdek 2013 Croatia; Martínez 2014 Spain; Santina 2011 Spain). Pre‐ban data for these studies were collected prior to the introduction of the national smoking ban, as smoking would have been banned in these settings after the introduction of legislation. Five studies assessed the impact in countries (Switzerland, US states) with no national smoke‐free legislation (Etter 2008; Etter 2012; Keizer 2009; Lechner 2012; Seo 2011). There was no evidence that effect sizes, pooled across settings, differed by the status of national legislation, but there was considerable heterogeneity within the studies included in the 'no national ban' subgroup (I² = 78%), so pooled effects are not reported (Analysis 4.3).
4.3. Analysis.
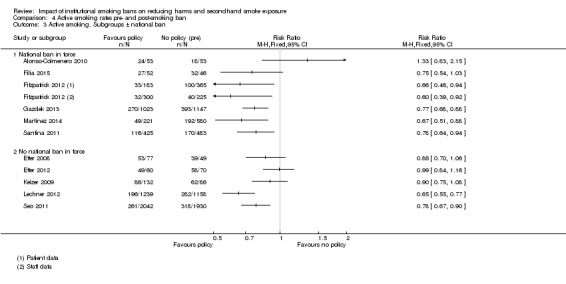
Comparison 4 Active smoking rates pre‐ and post‐smoking ban, Outcome 3 Active smoking. Subgroups ± national ban.
Adverse events
Four studies in the review reported adverse events during the period of the studies, with three studies specifically reporting events in psychiatric hospitals (Etter 2008; Filia 2015; Harris 2007) and Etter 2012 reporting events from the prison setting. Etter 2012 reported that smoking continued outdoors and in the cells of all prisons, due to a lack of enforcement of the smoking bans by staff.
In the hospital setting, Etter 2008 reported that after a total ban was introduced, both staff and patients significantly perceived that the smoking ban's rules were too strict and this perception increased with the ongoing progression of the smoking ban in the hospital (staff and patients: pre‐ban 9.4% versus 55.0% post‐total ban, P < 0.001). The total ban resulted in significantly more patients reporting that they became angry with staff because of the policy (4.5% partial ban versus 24.5% after total ban). However, whilst staff also reported a significant increase in patient complaints, this increase was not statistically significant. After the total ban was introduced, staff reported that patients were still smoking in bedrooms. There was an increase in patients reporting tobacco smoke as a source of conflict with staff (pre‐ban 24.7% versus 36.4% post‐total ban, P = 0.005), but a nonsignificant reduction reporting tobacco smoke as a source of conflict with other patients after the total ban was introduced (pre‐ban 49.0% versus 37.7% post‐total ban). However, the follow‐up period after the introduction of the total ban was short, at approximately three months, and the authors suggest this time period may not reflect acceptability.
Filia 2015 reported 75% of smokers held negative or very negative views about the introduction of a total smoke‐free policy in comparison with 7.1% of nonsmokers in the hospital. Smokers viewed smoking as a coping strategy for stress and were concerned about nicotine withdrawal symptoms. Patients reported difficulties including negative emotions, frustration, restlessness and anger with increased craving and symptoms of nicotine withdrawal. They reported drinking more tea and coffee. The authors suggest that reduced nicotine can increase caffeine levels and patients should be encouraged to limit caffeine intake, as high levels of caffeine can mimic the symptoms of nicotine withdrawal.
Harris 2007 reported negative health effects including increased prescribing for clozapine, reported cases of increased aggression and evidence of weight gain amongst inpatients in the study. The authors report that increased physical aggression by patients in the open wards was little to do with the tobacco ban as nonsmokers exhibited more aggression than those in maximum security and was probably due to selection, as only very violent patients would be inpatients for a long period of time. The ban was associated with a reduction in mood and increased weight gain of 5 kg, and with a decrease in clozapine dose. The authors report that the aggression levels returned to pre‐ban levels within a year, but weight gain was unchanged. They report that weight gains were no great than would be expected in the literature. The authors report that the increase in physical aggression was not seen in patients in maximum security units, and that few ill effects of the ban were observed in this group of patients. They suggest that the increased physical aggression in patients in open wards may have been due to staffing by nonforensic employees and less successful attempts at stopping tobacco use among patients, and also due to patients themselves having an opportunity to access tobacco from visitors or when they were off the hospital grounds while on recreation or work duties.
Discussion
Summary of main results
We included 17 studies in this review assessing the extent to which institutional smoking bans reduce active and passive smoke exposure and their effects on health‐related outcomes in a number of countries. Tobacco control bans and policies included in this review ranged from partial smoke‐free, which included indoor designated smoking areas, to comprehensive smoke‐free, banning smoking indoors, to complete tobacco‐free bans where tobacco products could not be bought or used indoors or outdoors. Extensions of smoke‐free policies were not limited to jurisdictions with national legislative bans. Eight studies included in this review were located in countries with no national legislative smoking bans.
Reductions in active smoking rates in staff were observed following the introduction of a smoking policy in two of the specialist settings (prisons, universities), as well as in patients in the hospital setting. There was also an increase in quit attempts and increased prescribing of NRT products in some studies. However, bias and the possibility of confounding are acknowledged, and we judge the overall quality of the evidence to be low. Reduced passive smoke exposure was also reported; however, there was no biochemical validation and we found inconsistencies in the implementation of policies within prison settings. Whilst smoke‐free prison policies have been increasing in US prisons and evidence of reduced mortality for smoking‐related illness identified, the implementation of such tobacco control bans and policies is limited in other jurisdictions. However, a ban on smoking in prisons in England and Wales will begin in 2016 (Ministry of Justice 2015).
Discrepancies in the types of smoke‐free bans being introduced in prisons continue within the European Union (EU). The findings in this current review provide evidence emerging from a USA study over a 10‐year period, where smoke‐free bans have been implemented with tobacco‐free bans evolving (Binswanger 2014). Fitzpatrick 2012 and Gazdek 2013 report improved outcomes for staff in particular after policies had been in place over a longer period of time. There seems therefore to be scope for implementing significant improvements to reduce passive smoke exposure in specialist settings.
Prisons and psychiatric hospitals were specialist settings exempted from initial smoke‐free legislation in many international jurisdictions, despite the fact they are workplaces and have been identified as settings with higher smoking prevalence rates. In these settings, cigarettes have been reportedly used to calm and control, used as “stress relievers”, and within prisons tobacco can be viewed as a currency (Connell 2010). In 2005, O'Dowd 2005 reported risks to staff if smoking were to be banned in prisons; McCaffery 2012 acknowledged prison riots in Canada following the introduction of smoke‐free bans, but reported 79% of EU members had introduced a smoking ban in prisons (n = 22) in their paper. Smoking rates and tobacco consumption rates are higher in prisoners when compared to the general population, with estimates of around 64% to 88% (Hartwig 2008), and rates of smoking in those with mental illness ranging from 44% up to 64% (McManus 2010). Negative health outcomes and impacts of smoking have been acknowledged and are well documented (Royal College of Physicians 2013). McCaffery 2012 observed air quality measures in Irish psychiatric hospitals were broadly similar to those found in Irish bars prior to the 2004 smoke‐free legislative ban, with excessively high particulate concentrations detected in psychiatric hospitals and nursing homes.
MacKay 2016 acknowledges the introduction of smoking bans in enclosed areas (prisons, psychiatric hospitals) is contentious; however, the rights of smokers to smoke has not been upheld in the courts under Human Rights legislation (Christie 2014). The reduction in harm and improvement in meeting public health goals is paramount. However, the introduction of bans in these areas must be sensitive to populations and be introduced as part of a multi‐component range of tobacco control measures to support smoking cessation. For example, it is important that bans in psychiatric hospitals are introduced in consultation with psychiatrists, ensuring that the best interests and improved health outcomes of patients are paramount. This would be in keeping with the UK NICE guidelines (NICE 2013), the Framework on Tobacco Control (WHO 2003), and ongoing progress being made (WHO 2014).
The importance of staff attitudes and their experiences are acknowledged as essential factors in enacting smoke‐free policies. Lawn 2015 acknowledges that attitudes of staff in prisons are important in the enforcement of smoke‐free policies. In this current review, Etter 2012 described staff authorising smoking in non‐designated areas in prisons, similarly to Lawn 2015’s review.
Evidence has emerged of reduced mortality from smoking‐related illnesses in prison populations. The association is consistent with a temporal dose response as the number of bans increased over time. However, the findings are limited to US studies, and the impact of other confounders, including changed prison diets, may have influenced the reported outcomes. Limited evidence exists of other health impacts at the settings level. The evidence in this review is limited to one study identifying reduced trends in AMI rates in inpatients and improved health assessments in a cohort of patients with enduring mental illness.
The implementation of university campus smoke‐free bans and policies in two studies in this review present evidence of a positive effect of introducing smoking bans, including reduced active smoking rates, increased quit attempts, evidence of reduced passive smoke exposure and positively influencing social norms and peer perceptions of smoking attitudes and behaviours.
Overall completeness and applicability of evidence
The key purpose of this review was to assess the extent to which institutional smoking bans may reduce active smoking and tobacco consumption, passive smoke exposure, and the effects on health‐related outcomes in three specialist settings, including public healthcare facilities, higher education and correctional facilities. We found 17 studies in total; however, only two addressed the question in the higher educational setting, and three in prisons. The majority of studies in this review provide evidence from public healthcare facilities. The evidence emerged from eight countries; however, the USA, Spain and Switzerland account for 12 of the 17 studies in this review.
Quality of the evidence
The quality of evidence included in this review is low, primarily reported from observational, uncontrolled before‐and‐after study designs. Only three studies employed a control location for comparison. Confounding, including the impact of other anti‐smoking activities on smoking outcomes, therefore needs to be considered. The 17 studies included in this review are heterogeneous and include patient surveys, staff surveys, university student surveys, prisoner surveys, and a review of mortality data, health outcomes data, including clozapine prescribing, and inpatient AMI rates. Hospital settings included general hospitals, a cancer centre, and psychiatric hospitals. A number of the studies used small sample sizes or limited inferential statistical analyses (Filia 2015; Ripley‐Moffitt 2010), and only two studies used biochemical verification to identify smokers in their patient populations, the majority of studies using self‐reported smoking status. However, large data sets were used by Binswanger 2014 and Dickert 2015, and a number of studies included large survey samples. Overall our GRADE assessment and Table 1 identify the evidence in this review as low quality, due to the study designs employed. Our confidence in the effect estimate is limited, and the true effect may be substantially different from the estimate of the effect made here.
Potential biases in the review process
This extensive review is of three data sources, including the Cochrane CENTRAL Register, MEDLINE and EMBASE. Language was not a limitation for this study, and two papers were translated for inclusion in this review (Alonso‐Colmenero 2010; Santina 2011). Similarly, our rationale to give preference to studies with a background national smoke‐free ban (including state or regional bans) post‐2005 is robustly policy‐based. Many bans were put in place in public areas first, progressing to implementation of smoke‐free bans in these specialist settings.
Reported meta‐analyses do not include all studies in this review. A pooled analysis is presented for 11 studies reporting active smoking outcomes measures. The resulting heterogeneity is acknowledged and we have tried to investigate this further.
Agreements and disagreements with other studies or reviews
The results in this review are, in part, consistent with those reported by Callinan 2010 and Frazer 2016 in their review of the effect of national smoking bans, which includes workplaces. However, limited evidence exists for the three specialist settings included in this review when compared to the studies described in Callinan 2010. Insufficient evidence of the effect of active smoking in the prison setting may be due to the initial exemption of prisons from smoking legislation and policies, identified by Ginn 2013. Similarly, limited evidence of the impact of tobacco control policies in schools was reported by Coppo 2014; their review included data from one cluster RCT. Our review includes nonrandomized, observational studies, as policy interventions may not be suitable for randomization methods when the outcome is smoking exposure and the intervention is a policy at a meso‐organizational level.
Authors' conclusions
Implications for practice.
In the 10 years since the introduction of national legislative indoor smoking bans, we found evidence of an effect of settings‐based smoking policies on reducing active smoking rates in hospitals and universities. The greatest reductions were observed amongst hospital‐based staff. In prisons, reduced mortality rates and reduced exposure to environmental tobacco smoke were reported. Increased quit attempts and evidence of support for tobacco control bans and policies occurred after policy implementations. However, the evidence assessing the impact of settings‐level tobacco control bans and policies is methodologically weak, and there are inconsistencies between specific participant groups.
Settings‐level tobacco control bans and policies could therefore be considered as components of multifactorial tobacco control activities to reduce passive smoking and reduce active smoking rates. However, taking the limitations of the literature into account, it is important that implementation is closely monitored to limit the impact of adverse events, and to ensure that the costs of implementing the intervention do not outweigh the benefits.
Implications for research.
There is a need for more robust studies assessing the impact of smoking bans and policies in these important specialist settings, to enable a settings‐based approach to health promotion and increased efforts to reduce the impact of passive smoke exposure. Future studies should use a control group for comparisons and robust biochemically‐measured outcomes. Better‐documented studies reporting both pre‐ and post‐ban data are required, with longer follow‐up periods of at least six months, and ideally of longer duration.
Acknowledgements
We wish to acknowledge support and assistance, in particular with searches, from Dr Lindsay Stead and from all of the Cochrane Tobacco Addiction Review Group in the preparation and completion of this review. We acknowledge the assistance of Susan van Baarsel for her assistance with the screening of a number of titles and abstracts.
Appendices
Appendix 1. Search strategies
MEDLINE
1. Air Pollution/lj, pc [Legislation & Jurisprudence, Prevention & Control]
2. Tobacco Smoke Pollution/lj, pc [Legislation & Jurisprudence, Prevention & Control]
3. Air Pollution, Indoor/lj, pc [Legislation & Jurisprudence, Prevention & Control]
4. (clean adj1 air).ti,ab.
5. Smoke‐Free Policy/
6. ((smok* or tobacco) adj4 (ban or bans or banned or law or laws or policy or policies or prohibit* or restrict* or regulat* or legislat* or ordinance*)).ti,ab.
7. 1 or 2 or 3 or 4 or 5 or 6
8. Smoking Cessation/
9. "tobacco use"/ or "tobacco use cessation"/
10. Tobacco Smoke Pollution/
11. "Tobacco Smoke Pollution".ti,ab.
12. "environmental tobacco smoke".ti,ab.
13. ('second hand smoke' or 'secondhand smoke' or 'second‐hand smoke').ti,ab.
14. (passive adj3 smok*).ti,ab.
15. (smok* adj3 involuntary).ti,ab.
16. smoking cessation.ti,ab.
17. (smok* adj3 (quit* or stop* or ceased or abstain* or abstin* or prevent*)).ti,ab.
18. tobacco consumption.ti,ab.
19. (smok* adj3 prevalence).ti,ab.
20. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19
21. hospital*.mp.
22. exp Hospitals/
23. (clinic or clinics).mp.
24. Prisons/
25. prison*.mp.
26. Military Personnel/
27. Universities/
28. (university or universities).mp.
29. (college or colleges).mp.
30. 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29
31. 7 and 20 and 30
EMBASE
1. smoking regulation/
2. smoking ban/
3. ((ban or bans or banned or law or laws or policy or policies or prohibit* or restrict* or regulat* or legislat*) adj4 (smok* or tobacco)).ti,ab.
4. 1 or 2 or 3
5. smoking cessation/
6. smoking/
7. passive smoking/
8. indoor air pollution/
9. cigarette smoke/
10. "Tobacco Smoke Pollution".ti,ab.
11. "environmental tobacco smoke".ti,ab.
12. ('second hand smoke' or 'secondhand smoke' or 'second‐hand smoke').ti,ab.
13. (passive adj3 smok*).ti,ab.
14. (smok* adj3 involuntary).ti,ab.
15. smoking cessation.ti,ab.
16. (smok* adj3 (quit* or stop* or ceased or abstain* or abstin* or prevent*)).ti,ab.
17. tobacco consumption.ti,ab.
18. (smok* adj3 prevalence).ti,ab.
19. 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18
20. exp hospital/
21. hospital*.mp.
22. cancer center/ or community mental health center/ or mental health center/ or rehabilitation center/ or residential home/ or secondary care center/ or tertiary care center/
23. (clinic or clinics).mp.
24. exp prison/
25. prison*.mp.
26. army/
27. university/
28. (university or universities).mp.
29. college/ or community college/
30. (college or colleges).mp.
31. 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30
32. 4 and 19 and 31
Data and analyses
Comparison 1. Passive exposure (narrative).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Passive smoke exposure | Other data | No numeric data |
Comparison 2. Health and mortality outcomes (narrative).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Health and mortality outcomes | Other data | No numeric data |
Comparison 3. Active smoking (narrative).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Active smoking rates | Other data | No numeric data |
Comparison 4. Active smoking rates pre‐ and post‐smoking ban.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Active smoking. Subgroups by setting | 11 | 12485 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.70, 0.79] |
| 1.1 Hospitals | 8 | 5986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.69, 0.81] |
| 1.2 Universities | 2 | 6369 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.64, 0.80] |
| 1.3 Prisons | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.84, 1.16] |
| 2 Active smoking. Hospital setting, staff/patients subgroups | 8 | 5986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.69, 0.81] |
| 2.1 Staff | 4 | 4544 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.64, 0.78] |
| 2.2 Patients | 5 | 1442 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.76, 0.98] |
| 3 Active smoking. Subgroups ± national ban | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 National ban in force | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 No national ban in force | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alonso‐Colmenero 2010.
| Methods | Country: Spain, Madrid Setting: Hospital Design: Uncontrolled before‐and‐after study Analysis: Chi² test, t‐tests, Mann‐Whitney and multivariate logistic regression analyses |
|
| Participants | 1189 patients admitted during June and July 2005 (pre) and June and July 2006 (post). 184 current smokers identified (15.4%). 135 smokers agreed to participate in study: completed survey of smoking habits, social status and consumption of snuff. Pre‐ban n = 73, post‐ban n = 62 n = 55 smokers actively smoked in hospital during study period Aged: 18 years and older |
|
| Interventions | National smoke‐free ban 2nd August 2005. Commenced in hospital 26th December 2005. Hospital policy not described. National policy not described | |
| Outcomes | Effect of smoking ban on smoking prevalence of inpatients during hospital stay Variables associated with tobacco use in hospital |
|
| Notes | National Ban: Yes National indoor smoking ban enacted 1st January 2006 banned direct and indirect tobacco publicity and sponsorship, it reduced points of sale, and it banned smoking in enclosed workplaces and public spaces, with exemptions in the restaurant and hospitality sector (Partial ban at time of study). Biochemical verification: CO‐oximetry Follow‐up period: 12 months Smokers were considered to be patients who admitted to smoking in the hospital ( or using snuff) and/or those with a CO‐oximeter result > 6 ppm Translated paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not applicable |
| Allocation concealment (selection bias) | High risk | Not applicable |
| Sampling bias (selection bias) | Unclear risk | Volunteers agreeing to participate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not applicable |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported smoking status |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unknown. Data for 135 smokers only |
| Selective reporting (reporting bias) | Unclear risk | Smokers combined for analysis vs nonsmokers and not pre‐ and post‐ban analysis |
| Other bias | High risk | Small sample size Recruitment from small number of clinics Other antismoking activities ongoing |
Binswanger 2014.
| Methods | Country: USA Setting: State prisons Design: Controlled before‐and‐after study. ITS mortality data Intervention: States with smoking bans Control/Comparison: States without smoking bans Analysis: Multivariate Poisson time series analysis |
|
| Participants | Analysis of self‐reported smoking data from Bureau of Justice Statistics. Nationally representative survey of individuals in state prisons from 2004. 297 prisons selected, 2 refused and 12 were "out of scope". 4 reserve female prisons added. Data on tobacco control policies for 50 sites from websites and American Non Smokers Rights Foundation 287 US state prisons participated, with a total of 14,499 individuals Several sources of data were used. Health survey from 2004 Department of Justice Data on deaths in custody from 2001 to 2011 stratified by age and sex Data were recorded for year end number of prisoners estimates by sex and age group Data were collected on tobacco control policies in all 50 states from 2001 to 2011 (primary exposure of interest) Data on smoking population in the general population collected to assess potential confounding |
|
| Interventions | Enactment varied by state, ordinance. Either smoke‐free (indoor ban), comprehensive (indoor and outdoor) or tobacco‐free policy | |
| Outcomes | Association between smoking bans and smoking‐related cancer, cardiovascular, and pulmonary deaths Measure smoking‐attributable mortality and years of potential life lost |
|
| Notes | National Ban: Varied by state Biochemical Verification: None. Mortality data and self‐reported smoking prevalence survey data Follow‐up period: 10 years Did not include deaths in local jails ICD codes used for diagnosis. Autopsy in 66.2% of cases Current smoker: smoked at least 100 cigarettes in lifetime and smoked every day or some days of the week Former smokers had ever smoked at least 100 cigarettes but did not smoke when interviewed National smoking prevalence data taken from general population survey to assess confounding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not applicable |
| Allocation concealment (selection bias) | High risk | Not applicable |
| Sampling bias (selection bias) | Unclear risk | Nationally representative survey of individuals in state prisons. No random sampling. Female prisons added from reserve list. Census of deaths recorded in prison |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not applicable |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported smoking from 2004 survey. Data used from national sources |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Unclear risk | SHS exposure in prisons higher than population Poorly‐ventilated cells Movement of prisoners (transit) over time and may be in prisons with and without bans during periods Confounders and other causes of death |
Dickert 2015.
| Methods | Country: USA, New Jersey Setting: Prisons Design: Uncontrolled before‐and‐after study Analysis: Annual and semi‐annual mortality rates. Boot strap analysis of the correlations between tobacco sales and mortality rates using Proc Survey Select in SAS |
|
| Participants | 13 prisons, N = 23,000 prisoners Mortality rates for all and mortality rates for subgroup with mental illness assessed Review of records Jan 2005 to June 2014 Median total term for the NJDOC’s prisoners 6 yrs, median age is 34 yrs. 60% of prisoners are black, 23% white, 16% Hispanic and 1% Asian Persons placed on the special needs roster account for approx. 13% of the total prison population; this includes all prisoners with a serious mental illness |
|
| Interventions | NJDOC policy decision for tobacco‐free prisons, including grounds 2012 13th Feb 2013 policy to ban sales and use of all tobacco products for employees, visitors and prisoners enacted |
|
| Outcomes | Effect of tobacco‐free prison policy on mortality rates in all prisoners and in those with mental illness | |
| Notes | National Ban: Yes. New Jersey’s Smoke‐Free Air Act prohibits smoking in enclosed indoor spaces (2006). March 2010, an amendment banned the use of electronic smoking devices in indoor public places and workplaces and the sale to people 19 years and younger Biochemical Verification: No Follow‐up period: 1½ years |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not applicable |
| Allocation concealment (selection bias) | High risk | Not applicable |
| Sampling bias (selection bias) | Low risk | Data collection from a defined population |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not applicable, accessing national data set |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Expected outcomes reported |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Unclear risk | Aggregate data. No individual patient data No comorbidities No SHS exposure Consumption not accurate as cigarettes traded in prison Differences in length of sentences Transit of prisoners Change of diet to healthy heart between 2005 and 2007 implemented in prisons |
Etter 2008.
| Methods | Country: Switzerland, Geneva Setting: Hospital. 2 psychiatric inpatient units Design: Uncontrolled before‐and‐after study Analysis: Descriptive and univariate analysis: Chi² analysis and odds ratios to compare proportions, independent t‐tests to compare means |
|
| Participants | Patients and staff completed anonymous self‐administered questionnaires. The survey was conducted from a sample of 2 inpatient adult units of the Psychiatry Department: short‐stay unit and medium‐stay unit Oct 2003: 106 ( baseline); patients n = 49; staff = 57 April 2004: 108 (2 months post‐ban); patients n = 54; staff n = 54 Oct to Dec 2005: 119 ( 20 months after partial smoking ban); patients n = 66; staff = 53 March to May 2006: 134 (3 ‐ 5 months after total smoking ban); patients n = 77; staff n = 57 Mean age: patients across surveys: 39.9 to 41.0 yrs Mean age: staff across surveys: 38.7 to 40.7 yrs |
|
| Interventions | Smoking prohibited in February 2004 everywhere except smoking rooms. January 2006 smoking rooms removed and smoking totally prohibited inside hospital | |
| Outcomes | Smoking behaviour (prevalence, quit rates, and consumption) Perceived exposure to SHS Annoyance from SHS Awareness and satisfaction with no‐smoking policy | |
| Notes | National Ban: No Biochemical Verification: No, self‐reported smoking status Follow‐up period: 25 ‐ 27 months after partial ban, 3 ‐ 5 months after total ban Ever smoked defined as ever smoked 100+ cigarettes in lifetime No ethical approval for baseline or first follow‐up survey |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not applicable |
| Allocation concealment (selection bias) | High risk | Not applicable |
| Sampling bias (selection bias) | Unclear risk | Sample of patients and staff |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not applicable |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐report smoking status. ETS exposure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Expected outcomes reported. Response rates reduced in patients over 4 surveys |
| Selective reporting (reporting bias) | Unclear risk | Additional questions on cessation activities asked in subsequent staff surveys |
| Other bias | Unclear risk | Staff may have participated in all surveys. Not linked and analysed as independent t‐tests Follow‐up period after total ban only 3 ‐ 5 months (but up to 27 months after first ban) Enforcement of ban SHS exposure unknown Other confounders unknown Small sample size No control group No ethical approval for first 2 surveys |
Etter 2012.
| Methods | Country: Switzerland Setting: Prison Design: Controlled before‐and‐after study Intervention: Prison A open prison Comparison: Prison B: closed prison; Prison C : prison for pre‐trial detainees Analysis: Chi² tests used to compare proportions, Mann–Whitney U tests compare medians, independent‐sample t‐tests to compare means |
|
| Participants | Surveys of prisoners and staff Pre: 2009 Prison A: n = 70 male prisoners individual cells (response rate 58%). n = 51 staff ( response rate 43%) Open regimen and prisoners work outdoors, or indoor workshops. Prisoners have freedom of movement Prison B: n = 27 male prisoners, individual cells within walls ( response rate 40%). n = 27 staff ( response rate 77%) Prison C: Built for 270 detainees, almost all male. In 2009 housed 490, 560 in 2010, +400 in 2011. 2 or 3, but up to 6 prisoners per cell. Sample 2009 n = 116 ( response rate 23%). n = 126 staff ( response rate 54%) Follow‐up 13 months later in 2010/2011. 6 ‐ 9 months follow‐up in prisons A and C and only 3 months follow‐up in prison B Prison A: n = 60 (response rate 50%). n = 48 staff ( response rate 40%) Prison B: n = 30 (response rate 44%). n = 24 staff ( response rate 63%) Prison C: n = 66 ( response rate 17%). No follow‐up for staff 2011 Prison A most prisoners were Swiss citizens. Prison B: 5 ‐ 15% of prisoners were Swiss, Prison C: duration of imprisonment doubled between 2009 and 2011, 2.8 months to 5.4 months , P = 0.02 Proportion of prisoners housed unchanged over study period |
|
| Interventions | In prison A, the SHS intervention consisted of an extension of smoke‐free zones and in 2009 smoking allowed everywhere except some indoor workplaces. From 2010 smoking only allowed in cells and outdoors. In prisons B and C in 2009, prisoners were allowed to smoke only in cells, during their outdoor exercise and in 1 smoking room in prison C. Rules were loosely enforced and respected. There was no policy change regarding SHS in prison B. In prison C, the SHS intervention was limited to better enforcement of the smoking ban in the waiting rooms of the medical service No cessation programmes in Prison A, inmates charged for NRT, prison B and C in 2010/2011 medical staff trained to provide smoking cessation counselling and provide NRT. Prison C ‐ NRT was free in this prison only. Smoking cessation booklets distributed to all prisons |
|
| Outcomes | Self‐reported SHS levels Attitudes towards ban Smoking prevalence and tobacco consumption, quit attempts |
|
| Notes | National Ban: No Biochemical Verification: No, self‐reported smoking status Follow‐up period: 6 ‐ 9 months for prisons A and C. 3 months for prison B Questionnaires self‐administered and anonymous and available in 8 languages |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not applicable |
| Allocation concealment (selection bias) | High risk | Not applicable |
| Sampling bias (selection bias) | Unclear risk | Low response to survey |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not applicable. Prison questionnaires could be completed and returned or assistance from research assistants. Staff questionnaires were returned by mail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported smoking status |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Low response rates amongst prisoners |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Unclear risk | Sample sample sizes Movement of prisoners Self‐reported smoking status No measure of SHS exposure Confounders No follow‐up survey for staff in Prison C due to staff shortages |
Filia 2015.
| Methods | Country: Australia, Melbourne Setting: Hospital, acute psychiatric ward Design: Uncontrolled before‐and‐after study Analysis: Frequency and descriptive statistics. Chi² test for independence |
|
| Participants | Total sample (baseline and follow‐up): 98 patients (2 cross‐sectional surveys)
n = 46 inpatients completed a questionnaire assessing their views before the smoking ban. No demographics given
n = 52 inpatients completed questionnaire assessing their views and experiences after the smoking ban Men 57.7%, women 42.3% Age (Mean, SD): 39.1 (10.8) |
|
| Interventions | Total smoking ban implemented in the inpatient psychiatric unit in June 2008, including outdoor areas | |
| Outcomes | Patient tobacco consumption Smoking prevalence Attitudes towards ban |
|
| Notes | National Ban: Yes, State ban State ban Victoria: 1st July 2007. All restaurants, cafes, dining areas and shopping centres, enclosed workplaces, covered railway platforms, bus and tram stops and underage music and dance events are smoke‐free. Enclosed licensed premises and outdoor eating and drinking areas (where there is a roof and the wall surface area is more than 75%) must also be smoke‐free as of July 1, 2007. The gambling floors of casinos exempt. Biochemical Verification: No Follow‐up period: 7 ‐ 8 months Heavy smokers considered as those who smoked > 20 cigarettes/day. Light smokers those who smoked < 10 cigarettes/day Self‐reported smoking status |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not applicable |
| Allocation concealment (selection bias) | High risk | Not applicable |
| Sampling bias (selection bias) | Unclear risk | Inpatients asked to complete anonymous questionnaire by ward occupational health staff |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None. Anonymous surveys returned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported smoking status |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Response rates not reported |
| Selective reporting (reporting bias) | Unclear risk | Patient demographics details available for post policy survey |
| Other bias | High risk | Small sample size No control group |
Fitzpatrick 2012.
| Methods | Country: Ireland, Dublin Setting: Hospital Design: Uncontrolled before‐and‐after study Analysis: Chi² test was used for comparison of proportions and Student's t‐test for comparison of means Logistic regression |
|
| Participants | Pre‐national ban (reported in Fitzpatrick 2009) Census surveys patients (face‐to‐face interviews) 1997 and 1998, Sept 2002, July 2004 census interviews patients Proportional sampling Jan 1998 ‐ staff (face‐to‐face). 2001: (self‐administered questionnaires) Patients (pre‐ban) 1997/1998, n = 711 (combined) 2002, n = 329 Post‐national ban 2004 n = 259 Staff (pre‐ban) 1998,n = 365, 2001, n = 556 Cross‐sectional survey of patients and staff 2006 and 2010 reported in Fitzpatrick 2012. 2006 surveys were post‐national ban and pre‐2009 extension of ban to total campus ban. 2010 surveys were post‐national ban and post‐extension of total campus ban in 2009. Staff surveyed face‐to‐face or by telephone interview. Census survey of inpatients: eligible to participate (all inpatients with exception of day care and those too ill to participate). Written consent obtained prior to face‐to‐face interviews for all surveys. Staff: 2006: n = 225 2010: n = 300 Patients: 2006: n = 295 2010: n = 183 |
|
| Interventions | National ban on smoking indoors in public buildings, introduced in March 2004 Total smoke‐free hospital campus policy in 2009. No smoking permitted indoors or outdoors |
|
| Outcomes | Smoking prevalence of staff and patients Acceptance of campus ban, beliefs about passive smoking Smoke‐free area in home |
|
| Notes | National Ban: Yes, 2004 Smoking banned in general workplace, enclosed public places, restaurants, bars, education facilities, healthcare facilities and public transport. However, it is permitted in designated hotel rooms and there is no ban in residential care, prisons and in outdoor areas Biochemical Verification: Yes. Patients with CO levels > 10 ppm were considered to be current smokers in 2006 and 2010. Staff smoking self‐reported and not validated Follow‐up period: 12 months after total campus ban and 6 years after a national ban |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not applicable |
| Allocation concealment (selection bias) | High risk | Not applicable |
| Sampling bias (selection bias) | Unclear risk | Quota sampling of staff randomly selected representing 10% of staff from each occupational health group. Census survey of inpatients |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None. Face‐to‐face surveys, except in 2001 when questionnaires for staff were attached to payslips |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported smoking status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Expected outcomes reported |
| Selective reporting (reporting bias) | Unclear risk | Due to small sample size, non‐consultant doctors were merged with consultants to form "medical group" for sampling. Allied services staff and cleaning staff merged for analysis (Fitzpatrick 2012) |
| Other bias | Unclear risk | Other anti‐smoking activities 1997 and 1998 patient surveys were combined for reporting Response rates for staff survey in 2001 was 25% due to alternative administration Validated cotinine available for patient survey 2006 |
Gadomski 2010.
| Methods | Country: USA, New York Setting: Hospital Design: Uncontrolled before‐and‐after study (cohort of staff) Analysis: McNemar test and t‐tests |
|
| Participants | Employee tobacco use rates from occupational health assessments accessed Percentage patients smoking and NRT orders obtained from electronic records. Number of inpatients who signed out against medical advice obtained from incident records A cohort of 489 staff were surveyed to determine smoking prevalence pre‐ and post‐ban and followed up. March to June 2005 pre‐ban and March to June 2006 and 2007. All hospital employees were also surveyed at 3 points ‐ total N not provided for all employees An average of 959 patients were admitted per month in the 18‐month period pre‐ban and 988 per month in the 23 month post‐ban |
|
| Interventions | Smoke‐free medical campus implemented on July 1, 2006, which included an NRT programme and additional signage | |
| Outcomes | Patient and employee smoking prevalence Percentage of inpatient NRT orders, number of inpatients who signed out against medical advice (obtained from incident reports) recorded | |
| Notes | National Ban: Yes, 2003 New York State Smoke‐free Air Act 2002, enacted 2003. Banned smoking in virtually all workplaces and indoor recreational venues. Amendment to the City’s 1995 Smoke‐Free Air Act, the new law banned smoking in all restaurants and most bars regardless of seating and size. The law restricted smoking in some outdoor restaurant and bar seating areas Biochemical Verification: No, staff self reported if smoked or chewed tobacco Follow‐up period: 1 year post‐policy. 2 years follow‐up for NRT prescribing (patients) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not applicable |
| Allocation concealment (selection bias) | High risk | Not applicable |
| Sampling bias (selection bias) | Unclear risk | Data collected on all patients from electronic records, all hospital staff surveyed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not applicable |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported smoking status. Data obtained from hospital records and annual employee assessment records |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Total number of staff employed not reported |
| Selective reporting (reporting bias) | Unclear risk | Only post‐ban smoking prevalence reported for patients |
| Other bias | Unclear risk | Cessation counsellor works part‐time and access limited No night admissions reviewed by counsellor Self‐reported smoking status No pre‐ban smoking prevalence data for patients |
Gazdek 2013.
| Methods | Country: Croatia, Koprivnica‐Krizevci County Setting: Hospital Design: Uncontrolled before‐and‐after study Analysis: Chi² test in Excel |
|
| Participants | Staff 4 surveys Pre‐ban 1998 n = 1147 staff (response rate 44%) Post‐hospital ban 2002 n = 1246 (response rate 50%) 2006 n = 1371 (response rate 44%) 2011 n = 1023 (response rate 68%) |
|
| Interventions | National ban: Yes, 2008 1999: smoking officially banned in government buildings, private worksites, educational and healthcare facilities, taxis, and domestic or international air flights. Smoking restricted (not banned) on trains, ferries, restaurants, nightclubs and bars, and other public places (Hrbak‐Zerbajic 2004). National legislative indoor smoking ban adopted 2008 |
|
| Outcomes | Smoking status Quit attempts |
|
| Notes | National Ban: Yes, 1999 (partial ban), national legislative ban adopted 2008 Ban 1999: (as noted above). November 2008: extension of ban in any and all public places. This is not reported in paper. Biochemical Verification: No, self‐report smoking status Follow‐up period: 2, 6, 11 years |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not applicable |
| Allocation concealment (selection bias) | High risk | Not applicable |
| Sampling bias (selection bias) | Unclear risk | Questionnaires handed to employees. Low response |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Anonymous questionnaires returned to researchers |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported smoking status |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Response rates varied 44% ‐ 68%. Quit attempts not reported. Totals not reported for trends, only percentages. No data reported for ex‐, non‐ and never‐smoker groups |
| Selective reporting (reporting bias) | Unclear risk | Quit attempts not reported. No significance testing |
| Other bias | Unclear risk | Limitions not discussed 'Not health workers' included a wide variety of occupational groups: administrative and technical staff, economists, lawyers, computer staff, scientists, maintenance, cleaners, ancillary and accountants Other anti‐smoking activities ongoing at time including extended national ban 2008 Self‐reported smoking |
Harris 2007.
| Methods | Country: Canada, Ontario, Penetanguishene Setting: Mental Health Centre Pentaguishene (MHCP) Design: Uncontrolled before‐and‐after study (cohort) Analysis: Analyses of variance, Fisher's exact test |
|
| Participants | Retrospective review of clinical notes and prescribing. Maximum‐security forensic mental health centre. Over 2 years (May 2002 to May 2004), 119 inpatients remained. n = 83 in the maximum security division and n = 32 in the open wards (the home for 4 patients was not recorded). The patients (89% male) had a mean age of 46.8 (SD = 11.1) years, with primary diagnoses of schizophrenia (47%), affective and other psychoses (14%), personality disorder (17%), mental retardation (12%), and unspecified and other disorders (10%) |
|
| Interventions | Comprehensive hospital tobacco ban. Tobacco products no longer allowed anywhere on 225‐acre grounds after May 6, 2003 | |
| Outcomes | Impact of tobacco ban on clozapine and olanzapine prescribing, patient mood, weight, number of adverse incidents Health assessment: cardiovascular health |
|
| Notes | National Ban: No. Ontario ban commenced in 2006 Ontario's Tobacco Control Act in 1994 banned smoking in all government buildings. Large psychiatric facilities, including MHCP, sought and received special dispensation to allow patients and some staff to smoke in specially ventilated rooms. Smoking rooms" were already in existence on most wards and some common patient areas at MHCP. The hospital constructed smoking gazebos outside various buildings for patients and staff to use Biochemical Verification: No. Smokers and nonsmokers identified from charts Follow‐up period: 1 year Retrospective review of hospital notes No smoking prevalence data reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not applicable |
| Allocation concealment (selection bias) | High risk | Not applicable |
| Sampling bias (selection bias) | Unclear risk | Retrospective cohort identified from clinical notes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not applicable |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Pre‐ban data extracted retrospectively from notes after policy was in place |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unknown |
| Selective reporting (reporting bias) | Unclear risk | Limited health assessment outcomes reported |
| Other bias | Unclear risk | Smoking status reported from chart data SHS exposure unknown Retrospective audit of notes Location of 4 inpatients not identified (open ward or maximum security) |
Keizer 2009.
| Methods | Country: Switzerland, Geneva Setting: Psychiatric Hospital Design: Uncontrolled before‐and‐after study Analysis: Chi² analysis, Fisher's exact test |
|
| Participants | Pre‐ post‐ban cross‐sectional survey of patients and staff. 7 of 9 inpatient units participated in study Staff questionnaires were anonymous and self‐reported. Patients invited to participate by care giver and where possible interviewed on 3rd day following admission and then on 10th day (2005 survey) 2001 n = 91/167, 54.49% inpatients and n = 110/281, 39.14% staff (Keizer ref 2005) 2005 n = 134/263, 51.14% inpatients and n = 85 /160 staff, 53.12% Inpatients aged 18 ‐ 60 years No significant differences in gender were recorded between the 2001 and 2005 surveys; however, patients were younger in 2001 and more nurses were surveyed in 2005 Pre‐policy survey November 2001, post‐policy study period Oct 2005 to January 2006 |
|
| Interventions | A partial smoking ban established in a psychiatric university hospital, where only 1 ventilated room was made available for smoking for inpatients. Indoor smoking was comprehensively banned for staff January 2002 | |
| Outcomes | Impact of a partial smoking ban on smoking and smoking‐related perceptions | |
| Notes | National Ban: No Biochemical Verification: No. For patients, tobacco consumption was measured before and after (3 days in 2001, 10 days in 2005) admission. Present daily consumption for staff was measured by asking about smoking habits in past week Follow‐up period: 3 years Current smoker: at least 100 cigarettes in lifetime/at least 1 cigarette per day during 6 months/ smoking during period of survey. Never‐smoker: less than 100 cigarettes during lifetime. Former smoker: at least 100 cigarettes in lifetime/at least 1 cigarette per day during 6 months/not smoking during period of survey. Occasional smoker or ex‐smoker: at least 100 cigarettes in lifetime/has never smoked every day during a period of 6 months or more (WHO criteria) 2 non‐participating units did not differ from 7 included. Patients were unavailable due to alternative research |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not applicable |
| Allocation concealment (selection bias) | High risk | Not applicable |
| Sampling bias (selection bias) | Unclear risk | Low response from staff and patients |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported smoking status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data from 1 unit excluded as smoking conditions differed (total smoking ban in place) |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Unclear risk | Self‐reported smoking status SHS unknown Staff and patients with chronic conditions could have participated in both surveys (unlinked) Patient participation reduced in 2005 and may be result of longer interview (and other questions on depression, anxiety and motivation scales for smoking ‐ not reported) Staff participation rates higher in 2005 as presentation on study prior to distribution of questionnaires |
Lechner 2012.
| Methods | Country: USA, Oklahoma Setting: University Design: Uncontrolled before‐and‐after study Analysis: Chi², ANOVA |
|
| Participants | 4 cross‐sectional surveys over 4 years pre‐ and post‐ban. Likert scale 6 points SHS exposure, 7‐point Likert scale for attitudes and beliefs. Cluster sampling of undergraduate students. Undergraduate courses selected were picked at random from a Registrar's list. Informed consent in group Participants enrolled in university between 2007 and 2010. Aged 18 ‐ 44 years Sample: 4947 undergraduate students. Baseline N = 1185 , 2008 N = 1197, 2009 N = 1257, 2010 N = 1242 Ages ranged from 18 ‐ 44 , mean age 20.3 yrs Women constituted 52.5% of the sample; 82.8% self‐identified as white, 4.1% as African‐American, 2.3% as Asian, 6.0% as Native American, and 2.3% as other Sex and ethnic distributions were typical of the campus |
|
| Interventions | 100% Tobacco‐free campus policy introduced in July 2008. The use, sales and promotion of tobacco products was prohibited | |
| Outcomes | Self‐reported prevalence Self‐reported exposure to SHS over 1 week period outside buildings and in walkways Attitudes to ban and to smoking | |
| Notes | National Ban: No Biochemical Verification: No, self‐reported smoking status Follow‐up period: 2 years follow‐up 4 groups were formed. Nonsmokers are classified as individuals who have not smoked over 100 cigarettes in their lifetime and have not smoked in the last 30 days. Former smokers are classified as individuals who have smoked at least 100 cigarettes in their lifetime but not in the last 30 days. Less frequent smokers are individuals who have smoked 1 ‐ 9 days in the last 30 (no cap on lifetime number). More frequent smokers are individuals who have smoked over 100 cigarettes and who consumed cigarettes on at least 10 of the last 30 days. Used CDC definitions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not applicable |
| Allocation concealment (selection bias) | High risk | Not applicable |
| Sampling bias (selection bias) | Unclear risk | Undergraduate courses were selected at random from Registrar's list. Explanation of randomization not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not applicable |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported smoking |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Expected outcomes reported |
| Selective reporting (reporting bias) | Unclear risk | Analysis limited to 2 groups containing active smokers |
| Other bias | Unclear risk | Self‐reported smoking status No control group and unclear whether downward trend in smoking related to policy or anti‐smoking activity |
Martínez 2014.
| Methods | Country: Spain Setting: Hospital, Cancer centre Design: Uncontrolled before‐and‐after study Analysis: Logistic regression to compare differences in the odds of smoking after the laws took effect |
|
| Participants | 6 cross‐sectional surveys from 2001 to 2012 Employees of oncology centre surveyed Total: n = 1263 Baseline: n = 580 After 1st law (2006 ‐ 2009): n = 462 After 2nd law (2012): n = 221 Female:male ratio remained stable at 75:25; however, the proportion of staff ≥ 35 years increased during study period. The professional status distribution also changed, with nurses accounting for 44.9% at baseline and 34.9% after extension of ban |
|
| Interventions | Smoke‐free centre policy was progressively introduced. Tobacco control programme (2000 ‐ 2012) National Ban 2005 (indoor smoking) National Ban 2011 (outdoor smoking) | |
| Outcomes | Attitudes to active and passive smoking Attitude to tobacco policies and restrictions Tobacco consumption and smoking status, quit attempts Staff compliance with policy |
|
| Notes | National Ban: Yes, 2005. Enacted 2006 2006 ‐ 2010, Spain had a partial ban on smoking in public places. Offices, schools, hospitals and public transportation were smoke‐free, but restaurants and bars could create a "smokers' section" or allow smoking if they were small (under 100 m²). Extension of ban January 2011 restricted smoking in every indoor public place, including restaurants, bars and cafes. Hotels may designate up to 30% of rooms for smoking; mental hospitals, jails and old people's residences may have public rooms where workers cannot enter. Outdoor smoking is also prohibited at childcare facilities, in children's parks and around schools and hospital grounds Biochemical Verification: No, self‐reported smoking status Follow‐up period: 1 year after full ban and 5 years after partial ban Face‐to‐face interviews by trained interviewers. Questionnaire developed European Network of smoke free hospitals Current tobacco consumption status as smokers either daily (at least 1 cigarette/day) or occasional smokers, former smokers, and never‐smokers as < 1 cig/day, former smokers (not smoking for ≥ 6 months)], and never‐smokers. Among daily smokers, tobacco dependence was evaluated in terms of the number of cigarettes per day (< 10, 10 – 20, and > 20) and the time to the first cigarette after waking up (≤ 30 and > 30 minutes) Studies all completed April to June periods Sample size calculation to account for smoking prevalence in health professionals in Catalonia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not applicable |
| Allocation concealment (selection bias) | High risk | Not applicable |
| Sampling bias (selection bias) | Unclear risk | Random sample of workers based on age and sex drawn from HR department updated files |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported smoking status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Expected outcomes reported |
| Selective reporting (reporting bias) | Unclear risk | Expected outcomes reported |
| Other bias | Unclear risk | Cancer centre and smoking reduction could be higher No biochemical measures of smoking status SHS exposure unknown No control |
Morito 2015.
| Methods | Country: Japan, Fukuoka Setting: Hospital Design: Uncontrolled before‐and‐after study Analysis: Unpaired t‐tests, significance of differences between mean values |
|
| Participants | Before‐and‐after study of in‐hospital AMI onset before and after smoking ban 2002 to 2014 Stage 1 2003 ‐ 2006 n = 14 Stage 2 2007 ‐ 2010 n = 4 Stage 3 2011 ‐ 2014 n = 3 Patients registered on hospital database under care of Cardiology or Emergency Departments. 25 patients with in‐hospital onset of AMI 2002 ‐ 2014 identified. Men: 16, women: 9. average age: 70 years, % hypertension 48%, % diabetes 48%, dyslipidaemia 56%. 6 died and 19 survived |
|
| Interventions | 2002 ‐ 2006 introduced smoke‐free zones in hospital. Smoking areas and smoking tables subsequently removed. Hospital became smoke‐free (indoors) in 2007 | |
| Outcomes | Effect of smoke‐free ban on incidence of in‐hospital AMI | |
| Notes | National Ban: No Biochemical Verification: No, self‐reported smoking status Follow‐up period: 3 time periods between 2002 and 2014 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not applicable |
| Allocation concealment (selection bias) | High risk | Not applicable |
| Sampling bias (selection bias) | Unclear risk | AMI cases identified from hospital database |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Dataset of admissions and self‐reported smoking status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Small sample size Self‐reported smoking status SHS exposures unknown Other confounders |
Ripley‐Moffitt 2010.
| Methods | Country: USA, North Carolina Setting: University‐affiliated Hospital Design: Uncontrolled before‐and‐after study (cohort) Analysis: Descriptive statistics |
|
| Participants | Before‐and‐after cohort study of staff Questionnaires: baseline: 2 questions on attitudes to policy and smoking prevalence. Current smokers or those who quit within previous 6 months were invited to participate in study about impact of policy on smoking behaviour Follow‐up questionnaires 6 months and at 1 yr Total sample N = 5534 full‐time employees with email addresses from hospital payroll database. Non‐responders followed up 3 days and 1 week N = 2024 respondents to initial survey (37%) of 2 questions. 247 employees (12%) currently smoked and 60 (3%) quit in past 6 months were invited to participate in study 210 employees (68%) enrolled into study. Mean age 42 yrs (SD 10). 82% women, white 73% Follow‐up 6 months 79% and 12 months 74% |
|
| Interventions | Tobacco‐free hospital policy introduced 4 July 2007. Employees offered free NRT, signage posted up and no‐smoking advertising 1 yr. lead in to policy. 100% tobacco‐free campus | |
| Outcomes | Self‐reported prevalence and quit attempts | |
| Notes | National Ban: No Biochemical Verification: No Follow‐up period: 6 and 12 months 6% of employees had no email addresses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not applicable |
| Allocation concealment (selection bias) | High risk | Not applicable |
| Sampling bias (selection bias) | High risk | Selection bias, as only 12% current smokers responded initially and reflected smoking prevalence 10% lower than State. Email address required. Incentives to participate in study USD 10 store card and USD 20 for each follow‐up |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported smoking |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition from study |
| Selective reporting (reporting bias) | Unclear risk | Reported outcomes not clearly reported |
| Other bias | Unclear risk | Quit line and advertising during period and other cessation services available during study period 16% employees had no email addresses Cessation and quit attempts not validated No control group |
Santina 2011.
| Methods | Country: Spain, Barcelona Setting: Hospital Design: Uncontrolled before‐and‐after study Analysis: Descriptive analysis |
|
| Participants | Pre‐/post‐ cross‐sectional surveys 2004 and 2007. Used European smoke‐free hospital network questionnaire 24 questions. Random sample of staff stratified by age, gender and occupation from population of 4077. 4% accuracy and 95% CI. Interviewer‐administered (medical students). Same methodology for both surveys. 483 staff members at baseline 2004. Women: 68.9% Men: 31.1%. Age: 42.69 (SD 10.57) 425 staff post‐ban 2007. Women: 69.2% Men: 30.8%. Age: 43.7 (SD 10.65) |
|
| Interventions | National smoking law introduced on January 1st 2006 and indoor smoking banned | |
| Outcomes | Smoking prevalence amongst staff | |
| Notes | National Ban: Yes. Enacted 1 Jan 2006 Spain had a partial ban upon smoking in public places. Offices, schools, hospitals and public transportation were smoke‐free, but restaurants and bars could create a "smokers' section" or allow smoking if they were small (under 100 m²). Biochemical Verification: No, self‐reported smoking status Follow‐up period: 12 months Sample size calculation: Yes Translated paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not applicable |
| Allocation concealment (selection bias) | High risk | Not applicable |
| Sampling bias (selection bias) | Unclear risk | Random sample of staff stratified by age, gender and occupation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not applicable. Interviewer‐administered questionnaire. Hawthorne effect |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported smoking |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Expected outcomes reported |
| Selective reporting (reporting bias) | Low risk | Expected outcome reported |
| Other bias | Unclear risk | Different interviewers If staff member did not want to participate, they were replaced by another matched for age, sex, occupation Media, advertising as part of ban may have influenced staff Self‐reported smoking status |
Seo 2011.
| Methods | Country: USA, Indiana Setting: University Design: Controlled before‐and‐after longitudinal study Intervention: Indiana University Comparison/Control: Purdue University Analysis: t‐tests, Z tests, linear growth models using HLM 6.08 software to analyse variation in change trajectories. Full maximum likeliness fixed‐effect and variance components |
|
| Participants | Intervention: Indiana University. Matched control: Purdue University, La Fayette Fall 2007: 84 Indiana instructors and 67 in Purdue asked to permit surveys of students in classes. 73/84 Indiana and 55/67 agreed. Total of 3492 students invited to complete questionnaire: n = 2057 from Indiana, n = 1435 from Purdue. Response Indiana 1930/2057; Purdue 1336/1435 Fall period 2009: 77/87 Indiana and 54/67 Purdue instructors agreed to follow‐up survey. Total of 3455 students invited to complete questionnaire n = 2215 Indiana, n = 1240 Purdue. Response rate Indiana: 2042/2215, Purdue 1165/ 1240 A longitudinal panel established of volunteers for longitudinal surveys: 377 from Indiana (2007 survey) and 318 Purdue. (Provided email addresses). Eligible and legible emails 301 Indiana, 231 Purdue. Online surveys fall 2008, spring 2009 and fall 2009 Indiana panel: sample participated in all surveys: n = 170 Purdue panel: sample participated in all surveys: n = 128 Mean age 20 years |
|
| Interventions | Indiana university total campus ban commenced 1 January 2008: smoking prohibited in all indoor and outdoor areas on campus. Smoking prohibited in university vehicles but not prohibited in personal vehicles Purdue University in West La Fayette allowed smoking at distance of at least 30 ft. from university facilities during study period |
|
| Outcomes | Effect of a smoke‐free campus policy on college students' smoking behaviours and attitudes | |
| Notes | National Ban: No Biochemical Verification: No, self‐reported smoking status Follow‐up period: 1½ years Participants who had smoked more than 100 cigarettes in their lives and reported smoking everyday or some days were categorised as current smokers 2 students completed longitudinal questionnaires by email and in class. Were excluded from analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization not applicable. |
| Allocation concealment (selection bias) | High risk | None |
| Sampling bias (selection bias) | Unclear risk | Volunteer groups and those with email addresses |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported smoking |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rate from longitudinal panel |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Unclear risk | Baseline smoking rate at Purdue was lower (Comparison) Multiple comparisons of single data set could have increased Type 1 error Other anti‐smoking activities occurring Gift card incentive for remaining in longitudinal panel |
AMI: acute myocardial infarction CDC: Centers for Disease Control and Prevention ETS: environmental tobacco smoke
MHCP:Mental Health Centre Penetang uishene NJDOC: New Jersy Department of Corrections SHS: secondhand smoke
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| An 2015 | Follow‐up period not 6 months |
| Arack 2009 | Attitudes post‐ban |
| Badowski 2013 | Attitudes and compliance post‐ban |
| Baillie 2011 | Post‐ban data only |
| Ballbe 2011 | No evidence of ban in paper |
| Bloor 2006 | Post‐ban data |
| Brandon 2014 | Multiple measures of smoking cessation with follow‐up of 8 weeks |
| Braverman 2015 | Post‐ban only |
| Brinn 2014 | Abstract only. Outcomes attitudes and knowledge |
| Brown 2012 | Implementation from administrator source. Outcomes did not meet inclusion criteria |
| Bussetti 2006 | Post‐ban follow‐up 1 month |
| Callaghan 2007 | Post‐ban data |
| Chaaya 2013 | Post‐ban data |
| Chang 2010 | Post‐ban qualitative study |
| Chelet‐Marti 2011 | Post‐ban data |
| Cho 2014 | ETS post‐ban only |
| Connell 2010 | No pre‐ban data |
| Corcoran 2010 | Follow‐up 1 month after release and no pre‐ban data |
| Cormac 2010 | Follow‐up 4 months |
| Cropsey 2005 | Follow‐up not 6 months |
| Cropsey 2008 | Quit rates only |
| Cushen 2014 | Post ban follow‐up 3 months |
| Eby 2012 | Qualitative outcomes post‐ban |
| Erdal 2015 | Post‐ban |
| Fallin 2015 | Not pre‐/post‐ data, outcome not applicable |
| Fathallah 2012 | Post‐ban data |
| Fernández 2008 | Air quality data only |
| Flavahan 2010 | Feasibility study and outcomes did not meet inclusion criteria |
| Frank 2015 | Follow‐up 21 days |
| Gandhi 2011a | Post‐ban |
| Gandhi 2011b | Post‐ban only. Outcomes not specific to this review |
| Gavigan 2011 | Post national ban reviewing prisons. Cross sectional study. Not a review of a settings policy. |
| Glassman 2011 | Outcomes not relevant for this review (did not meet inclusion criteria) |
| Gleason 2012 | Outcomes not relevant to review (did not meet inclusion criteria) |
| Hamadeh 2013 | No evidence of ban implemented. 22 years between baseline and follow‐up surveys |
| Harris 2009 | Compliance with ban |
| Hehir 2012 | Qualitative post policy review |
| Hehir 2013 | Post ban data |
| Heng 2007 | Not clear if all patients have 6 months follow‐up |
| Hofstetter 2010 | Feasibility study. Outcomes did not meet inclusion criteria |
| Hollen 2010 | Post‐ban eva luation of having a policy. Outcomes did not meet inclusion criteria |
| Iglesias 2008 | Follow‐up period 3 months |
| Iida 2008 | Letter with post ban details. |
| Jalilvand 2010 | No ban |
| Jancey 2014 | Post‐ban only |
| Jindal 2013 | Qualitative policy initiatives |
| Jovicevic 2009 | Pre‐ban qualitative data |
| Kamath 2011 | Post‐ban |
| Kauffman 2011 | Not pre‐ban |
| Kaushik 2013 | Post‐ban air quality |
| Kazmi 2010 | Post‐ban |
| Kitabayashi 2006 | 3‐month follow‐up |
| Lasnier 2011 | Post‐ban data |
| Laszlo 2013 | SHS exposure only |
| Lawn 2014 | Follow‐up not clear. Post‐policy review |
| Lawrence 2008 | Outcome did not meet inclusion criteria (bullying) |
| Lee 2013 | Outcome did not meet inclusion criteria (cigarette butts only) |
| Lincoln 2009 | Post‐ban |
| Lotufo 2011 | Pre‐ban only |
| Lucas 2014 | Post‐ban only |
| Marin 2008 | Post‐ban |
| Marin 2013 | Post‐ban |
| Matthews 2005 | Feasibility study. Not pre and post policy. |
| Maurel‐Donnarel 2010 | Post‐ban only |
| Mesenge 2014 | Not pre‐/post‐ban |
| Michopoulos 2013 | Not pre‐/post‐ban. Monitoring of smoking avoidance measures |
| Mishra 2011 | No ban |
| Ohmi 2013 | Post‐ban data |
| Pagano 2015 | Completion of pre‐ban data collected after ban introduced |
| Poder 2012 | Outcome not health measure. Did not meet inclusion criteria |
| Principe 2013 | Post ban air quality data |
| Proescholdbell 2008 | Air quality |
| Ratschen 2008 | Not pre‐/post‐data |
| Ratschen 2009a | Qualitative, not pre‐/post‐data |
| Ratschen 2009b | Not pre‐/post‐data. |
| Ritter 2012 | Air quality data |
| Rossi 2012 | Air quality |
| Sabidó 2006 | Post‐ban only |
| See 2014 | Abstract only |
| Sheffer 2009 | Outcomes not relevant to this review. Did not meet inclusion criteria (Resistance) |
| Shetty 2010 | Reported outcomes did not meet inclusion criteria |
| Sosman 2010 | Post‐ban |
| Stockings 2014 | Post‐ban |
| Sureda 2014 | AIr quality data |
| Tarnoki 2013 | Air quality data |
| Thornley 2013 | Air quality data |
| Tripathy 2013 | Post‐ban |
| Unrod 2012 | Follow‐up 3 months |
| Vardavas 2009 | Post‐ban |
| Voci 2010 | Post‐ban feasibility study |
| Vorspan 2009 | Follow‐up not 6 months |
| Wheeler 2007 | Attitudes only |
| Wye 2014 | Compliance only |
| Xiao 2013 | Follow‐up period not 6 months. |
Differences between protocol and review
The protocol stated that we would not report pooled results for meta‐analyses resulting in an I² over 50%. However, we decided to report the results of the pooled main analysis despite I² results of 72% and 76%, as we investigated this heterogeneity and found a potential explanation for some of it (by splitting the studies by whether they reported staff or patient outcomes and by settings).
The protocol stated that we would only included studies measuring SHS exposure with measured cotinine levels. We did not find any studies, but we include studies that included self‐reported exposure to SHS among adults in addition to reporting secondary outcomes.
Contributions of authors
KF and CK wrote the first draft of the protocol and review and completed subsequent revisions. All authors contributed to either conceptualising of the protocol and/or provided comments on drafts.
Sources of support
Internal sources
-
UCD School of Public Health, Physiotherapy and Sports Science, Ireland.
Mr Jack McHugh received an 8‐week Summer Student Research award
External sources
-
Health Research Board, Ireland.
Dr Kate Frazer was awarded a Cochrane Training Fellowship CTF/2013/5 commenced 2014.
Declarations of interest
Dr Kate Frazer: Cochrane training fellow
Mr Jack McHugh: eight‐week Summer Student Research Award.
Ms Joanne Callinan: none known.
Professor Cecily Kelleher: none known.
New
References
References to studies included in this review
Alonso‐Colmenero 2010 {published data only}
- Alonso‐Colmenero MM, Diez Jde M, Alvarez FV, Oteyza CP. [Tobacco consumption in hospitalized patients before and after the anti‐tobacco law (28/2005)]. Revista Clinica Española 2010;210(5):216‐20. [DOI] [PubMed] [Google Scholar]
Binswanger 2014 {published data only}
- Binswanger IA, Carson EA, Krueger PM, Mueller SR, Steiner JF, Sabol WJ. Prison tobacco control policies and deaths from smoking in United States prisons: population based retrospective analysis. BMJ 2014;349:g4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dickert 2015 {published data only}
- Dickert J, Williams JM, Reeves R, Gara M, DeBilio L. Decreased mortality rates of inmates with mental illness after a tobacco‐free prison policy. Psychiatric Services 2015;66(9):975‐9. [DOI] [PubMed] [Google Scholar]
Etter 2008 {published data only}
- Etter M, Etter JF. Acceptability and impact of a partial smoking ban in a psychiatric hospital.. Preventive Medicine 2007;44(1):64‐9. [DOI] [PubMed] [Google Scholar]
- Etter M, Khan AN, Etter JF. Acceptability and impact of a partial smoking ban followed by a total smoking ban in a psychiatric hospital. Preventive Medicine 2008;46(6):572‐8. [DOI] [PubMed] [Google Scholar]
Etter 2012 {published data only}
- Etter JF, Ritter C, Christie DH, Kunz M, Rieder JP, Humair JP, et al. Implementation and impact of anti‐smoking interventions in three prisons in the absence of appropriate legislation. Preventive Medicine 2012;55(5):475‐81. [DOI] [PubMed] [Google Scholar]
Filia 2015 {published data only}
- Filia S, Gurvich C, Horvat A, Shelton C, Katona L, Baker A, et al. Inpatient views and experiences before and after implementing a totally smoke‐free policy in the acute psychiatry hospital setting. International Journal of Mental Health Nursing 2015;24(4):350‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Fitzpatrick 2012 {published data only}
- Fitzpatrick P, Gilroy I, Doherty K, Clarke A, Comerford D, Daly L, et al. Smoke free hospital campus: strong positive shift in attitudes post implementation but paradox in nursing and medical attitudes. Clinical Health Promotion 2012;2:12‐8. [Google Scholar]
- Fitzpatrick P, Gilroy I, Doherty K, Corradino D, Daly L, Clarke A, Kelleher CC. Implementation of a campus‐wide Irish hospital smoking ban in 2009: prevalence and attitudinal trends among staff and patients in lead up. Health Promotion International 2009;24(3):211‐22. [DOI] [PubMed] [Google Scholar]
- Gilroy I, Clarke A, Comerford D, Conlon G, Daly L, Doherty K, et al. Evaluation of patient smoking rates and attitudes towards a total hospital campus smoking ban, 1 year post introduction. Irish Journal of Medical Science 2011;180(Suppl 6):S217‐8. [Google Scholar]
- Gilroy I, Doherty K, Comerford D, Clarke A, Daly L, Fitzpatrick P, et al. Evidence that smoking bans affect smoking culture in a large Irish teaching hospital, St. Vincent's University Hospital 1998‐2011. Irish Journal of Medical Science 2012;181(Suppl 4):S109. [Google Scholar]
Gadomski 2010 {published data only}
- Gadomski AM, Stayton M, Krupa N, Jenkins P. Implementing a smoke‐free medical campus: impact on inpatient and employee outcomes. Journal of Hospital Medicine 2010;5(1):51‐4. [DOI] [PubMed] [Google Scholar]
Gazdek 2013 {published data only}
- Gazdek D, Samardzic S. Croatian smoke‐free law and smoking habits among employees of healthcare facilities in Koprivnica‐Križevci County. Croatian Medical Journal 2013;54(4):407‐10. [DOI: 10.3325/cmj.2013.54.407] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2007 {published data only}
- Harris GT, Parle D, Gagne J. Effects of a tobacco ban on long‐term psychiatric patients. Journal of Behavioral Health Services & Research 2007;34(1):43‐55. [DOI] [PubMed] [Google Scholar]
Keizer 2009 {published data only}
- Keizer I, Descloux V, Eytan A. Variations in smoking after admission to psychiatric inpatient units and impact of a partial smoking ban on smoking and on smoking‐related perceptions. International Journal of Social Psychiatry 2009;55(2):109‐23. [DOI] [PubMed] [Google Scholar]
Lechner 2012 {published data only}
- Lechner WV, Meier E, Miller MB, Wiener JL, Fils‐Aime Y. Changes in smoking prevalence, attitudes, and beliefs over 4 years following a campus‐wide anti‐tobacco intervention. Journal of American College Health 2012;60(7):505‐11. [DOI] [PubMed] [Google Scholar]
Martínez 2014 {published data only}
- Martínez C, Fu M, Martínez‐Sánchez JM, Anton L, Fernández P, Ballbè M, et al. Impact of a long‐term tobacco‐free policy at a comprehensive cancer center: a series of cross‐sectional surveys. BMC Public Health 2014;14:1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez C, Garcia M, Méndez E, Peris M, Fernández E. Barriers and challenges for tobacco control in a smoke‐free hospital. Cancer Nursing 2008;31(2):88‐94. [DOI] [PubMed] [Google Scholar]
Morito 2015 {published data only}
- Morito N, Miura S, Yano M, Hitaka Y, Nishikawa H, Saku K. Association between a ban on smoking in a hospital and the in‐hospital onset of acute myocardial infarction. Cardiology Research 2015;6(3):278‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ripley‐Moffitt 2010 {published data only}
- Ripley‐Moffitt C, Viera AJ, Goldstein AO, Steiner JB, Kramer KD. Influence of a tobacco‐free hospital campus policy on smoking status of hospital employees. American Journal of Health Promotion 2010;25(1):e25‐8. [DOI] [PubMed] [Google Scholar]
Santina 2011 {published data only}
- Santina M, Grau J, Agusti C, Torres A. Assessment of effectiveness of a plan against tobacco in a universitary hospital [Evaluación de la efectividad de un programa de lucha contra el tabaquismo en un hospital universitario]. Revista de Calidad Asistencial 2011;26(4):215‐20. [DOI] [PubMed] [Google Scholar]
Seo 2011 {published data only}
- Seo DC, Macy JT, Torabi MR, Middlestadt SE. The effect of a smoke‐free campus policy on college students' smoking behaviors and attitudes. Preventive Medicine 2011;53(4‐5):347‐52. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
An 2015 {published data only}
- An DT, Kibria N, Huy NV, Hai PT, Stillman F. Establishing smoke‐free hospitals in Vietnam: a pilot project. Global Public Health 2015;10 Suppl 1:S5‐20. [DOI] [PubMed] [Google Scholar]
Arack 2009 {published data only}
- Arack R, Blake H, Lee S, Coulson N. An evaluation of the effects of the smoking ban at an acute NHS trust. International Journal of Health Promotion and Education 2009;47:112‐8. [Google Scholar]
Badowski 2013 {published data only}
- Badowski G, Silbanuez J, David A, Mummert A, Whippy H. Evaluation of tobacco‐free policy at University of Guam. Cancer Research 2013;73:Abstract 1364. [DOI: 10.1158/1538-7445.AM2013-1364] [DOI] [Google Scholar]
Baillie 2011 {published data only}
- Baillie L, Callaghan D, Smith ML. Canadian campus smoking policies: investigating the gap between intent and outcome from a student perspective. Journal of American College Health 2011;59:260‐5. [DOI] [PubMed] [Google Scholar]
Ballbe 2011 {published data only}
- Ballbe M, Nieva G, Mondon S, Pinet C, Bruguera E, Salto E, et al. Tobacco control strategies in psychiatric services in Catalonia (Spain). European Psychiatry 2011;26(Suppl 1):abstract 45. [Google Scholar]
Bloor 2006 {published data only}
- Bloor RN, Meeson L, Crome IB. The effects of a non‐smoking policy on nursing staff smoking behaviour and attitudes in a psychiatric hospital. Journal of Psychiatric & Mental Health Nursing 2006;13(2):188‐96. [DOI] [PubMed] [Google Scholar]
Brandon 2014 {published data only}
- Brandon TH, Klesges RC, Ebbert JO, Talcott GW, Thomas F, Leroy K, et al. Preventing smoking initiation or relapse following 8.5 weeks of involuntary smoking abstinence in basic military training: Trial design, interventions, and baseline data. Contemporary Clinical Trials 2014;38(1):28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Braverman 2015 {published data only}
- Braverman MT, Hoogesteger LA, Johnson JA. Predictors of support among students, faculty and staff for a smoke‐free university campus. Preventive Medicine 2015;71:114‐20. [DOI] [PubMed] [Google Scholar]
Brinn 2014 {published data only}
- Brinn M, Carson K, Smith B. Evaluation of the hospital smoke‐free implementation policy: A cross‐sectional cohort analysis. Respirology 2014;19:40. [Google Scholar]
Brown 2012 {published data only}
- Brown E, Nonnemaker J, Federman EB, Farrelly M, Kipnis S. Implementation of a tobacco‐free regulation in substance use disorder treatment facilities. Journal of Substance Abuse Treatment 2012;42(3):319‐27. [DOI] [PubMed] [Google Scholar]
Bussetti 2006 {published data only}
- Bussetti A, Tacconi C, Stopponi R, Armadori M, Giovannelli G, Siracusa A, et al. Tobacco smoke and involuntary smoking among hospital workers after enforcement of "Legge Sirchia". [Italian]. Giornale Italiano di Medicina del Lavoro ed Ergonomia 2006;28:415‐7. [Google Scholar]
Callaghan 2007 {published data only}
- Callaghan RC, Brewster JM, Johnson J, Taylor L, Beach G, Lentz T. Do total smoking bans affect the recruitment and retention of adolescents in inpatient substance abuse treatment programs?. A 5‐year medical chart review, 2001‐2005. Journal of Substance Abuse Treatment 2007;33(3):279‐85. [DOI] [PubMed] [Google Scholar]
Chaaya 2013 {published data only}
- Chaaya M, Alameddine M, Nakkash R, Afifi RA, Khalil J, Nahhas G. Students' attitude and smoking behaviour following the implementation of a university smoke‐free policy: a cross‐sectional study. BMJ Open 2013;3:e002100. [DOI: 10.1136/bmjopen-2012-002100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2010 {published data only}
- Chang CC, Huang CL, Chen CY. The impact of implementing smoking bans among incarcerated substance users: a qualitative study. Evaluation & the Health Professions 2010;33(4):473‐9. [DOI] [PubMed] [Google Scholar]
Chelet‐Marti 2011 {published data only}
- Chelet‐Marti M, Escriche‐Saura A, Garcia‐Hernandez J, Moreno‐Bas P. [Tobacco consumption in a University of Valencia population]. Trastornos Adictivos 2011;13:5‐10. [Google Scholar]
Cho 2014 {published data only}
- Cho H, Lee K. Hwang Y, Richardson P, Bratset H, Teeters E, et al. Outdoor tobacco smoke exposure at the perimeter of a tobacco‐free university. Journal of the Air & Waste Management Association 2014;4(8):863‐6. [DOI] [PubMed] [Google Scholar]
Connell 2010 {unpublished data only}
- Connell A. Tobacco‐free prison policies and health outcomes among inmates. University of Kentucky Doctoral Dissertations. Paper 22 2010. [ uknowledge.uky.edu/gradschool_diss/22]
Corcoran 2010 {published data only}
- Corcoran K, Seal D, Thibodeau L, Jorenby D, Sosman J. Smoking intention, motivation, and behavior of men awaiting release from prison‐qualitative findings. Journal of General Internal Medicine 2010;25:S391. [Google Scholar]
Cormac 2010 {published data only}
- Cormac I, Creasey S, McNeill A, Ferriter M, Huckstep B, D'Silva K. Impact of a total smoking ban in a high secure hospital. Psychiatrist 2010;34:413‐7. [Google Scholar]
Cropsey 2005 {published data only}
- Cropsey KL, Kristeller JL. The effects of a prison smoking ban on smoking behavior and withdrawal symptoms. Addictive Behaviors 2005;30(3):589‐94. [DOI] [PubMed] [Google Scholar]
Cropsey 2008 {published data only}
- Cropsey K, Eldridge G, Weaver M, Villalobos G, Stitzer M, Best A. Smoking cessation intervention for female prisoners: addressing an urgent public health need. American Journal of Public Health 2008;98(10):1894‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cushen 2014 {published data only}
- Cushen B, Lukjanova K, Ahern E. The effect of the introduction of a tobacco‐free hospital policy on documentation of smoking status and prescription of Nicotine Replacement Therapy (NRT). Irish Journal of Medical Science 2014;183(Suppl 7):S335. [Google Scholar]
Eby 2012 {published data only}
- Eby LTT, Sparks TE, Evans E, Selzer JA. A qualitative examination of the positive and negative consequences associated with going tobacco‐free in substance abuse treatment: The NY state experience. Nicotine and Tobacco Research 2012;14(12):1407‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Erdal 2015 {published data only}
- Erdal G, Erdal H, Esengun K, Karakas G. Cigarette consumption habits and related factors among college students in Turkey: A logit model analysis. Journal of the Pakistan Medical Association 2015;65(2):136‐41. [PubMed] [Google Scholar]
Fallin 2015 {published data only}
- Fallin A, Roditis M, Glantz SA. Association of campus tobacco policies with secondhand smoke exposure, intention to smoke on campus, and attitudes about outdoor smoking restrictions. American Journal of Public Health 2015;105(6):1098‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fathallah 2012 {published data only}
- Fathallah N, Maurel‐Donnarel E, Baumstarck‐Barrau K, Lehucher‐Michel MP. Three‐year follow‐up of attitudes and smoking behaviour among hospital nurses following enactment of France's national smoke‐free workplace law. International Journal of Nursing Studies 2012;49(7):803‐10. [DOI] [PubMed] [Google Scholar]
Fernández 2008 {published data only}
- Fernández E, Fu M, Martínez C, Martínez‐Sánchez JM, López MJ, Martín‐Pujol A, et al. Secondhand smoke in hospitals of Catalonia (Spain) before and after a comprehensive ban on smoking at the national level. Preventive Medicine 2008;47(6):624‐8. [DOI] [PubMed] [Google Scholar]
Flavahan 2010 {published data only}
- Flavahan R, Alam F. Impact of the smoking ban on the incidence of adverse events in adult psychiatry wards on the Wirral. European Psychiatry 2010;25(Suppl 1):720. [Google Scholar]
Frank 2015 {published data only}
- Frank M, Min S J, Blumhagen R, Weitzenkamp D, Mueller S, Beaty B, et al. Tobacco use among former inmates: Relapse and predictors of a desire to quit. Journal of General Internal Medicine 2015;30:S283. [Google Scholar]
Gandhi 2011a {published data only}
- Gandhi AR, Majoor J, Lubman D, Katz PH, Segal J. Smoke‐free mental health units: a survey of mental health professional knowledge and attitudes. Australian and New Zealand Journal of Psychiatry 2011;45:A60. [Google Scholar]
Gandhi 2011b {published data only}
- Gandhi AR, Segal J, Majoor J, Katz PH. Experience of implementing smoke‐free mental health units with focus on inpatient units. Australian and New Zealand Journal of Psychiatry 2011;45:A34. [Google Scholar]
Gavigan 2011 {published data only}
- Gavigan A, Goodman P, Young K, Clancy L. Exposure of prison officers to Second Hand Smoke (SHS) in the workplace. Irish Journal of Medical Science 2011;180:S462‐3. [Google Scholar]
Glassman 2011 {published data only}
- Glassman TJ, Reindl DM, Whewell AT. Strategies for implementing a tobacco‐free campus policy. Journal of American College Health 2011;59(8):764‐8. [DOI] [PubMed] [Google Scholar]
Gleason 2012 {published data only}
- Gleason H A, Hobart M, Jellison M, Seward G, Bradley L. Implementing tobacco education and cessation services at a large community mental health center: Lessons learned. Journal of Dual Diagnosis 2012;8:140‐7. [Google Scholar]
Hamadeh 2013 {published data only}
- Hamadeh RR. Smoking behavior of Arabian Gulf University medical students: Impact of tobacco control policies and curriculum. Journal of the Bahrain Medical Society 2013;24:56‐61. [Google Scholar]
Harris 2009 {published data only}
- Harris KJ, Stearns JN, Kovach RG, Harrar SW. Enforcing an outdoor smoking ban on a college campus: effects of a multicomponent approach. Journal of American College Health 2009;58(2):121‐6. [DOI] [PubMed] [Google Scholar]
Hehir 2012 {published data only}
- Hehir AM, Indig D, Prosser S, Archer VA. Evaluation of a smoke‐free forensic hospital: patients' perspectives on issues and benefits. Drug & Alcohol Review 2012;31(5):672‐7. [DOI] [PubMed] [Google Scholar]
Hehir 2013 {published data only}
- Hehir A M, Indig D, Prosser S, Archer V A. Implementation of a smoke‐free policy in a high secure mental health inpatient facility: staff survey to describe experience and attitudes. BMC Public Health 2013;13:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heng 2007 {published data only}
- Heng C, Badner V, Clemens D, Mercer L, Mercer D. The relationship of cigarette smoking to postoperative complications from dental extractions among female inmates. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(6):757‐62. [DOI] [PubMed] [Google Scholar]
Hofstetter 2010 {published data only}
- Hofstetter V, Rohner A, Muller‐Isberner R. The implementation of a smoking ban in a Forensic Psychiatric Hospital: Is this feasible, reasonable, and legal?. [German]. Sucht 2010;56:423‐7. [Google Scholar]
Hollen 2010 {published data only}
- Hollen V, Ortiz G, Schacht L, Mojarrad MG, Lane GM Jr, Parks JJ. Effects of adopting a smoke‐free policy in state psychiatric hospitals. Psychiatric Services 2010;61(9):899‐904. [DOI] [PubMed] [Google Scholar]
Iglesias 2008 {published data only}
- Iglesias C, Lopez G, Alonso M J. Effects of smoking ban in a general hospital psychiatric unit. Actas Españolas de Psiquiatría 2008;36(1):60‐2. [PubMed] [Google Scholar]
Iida 2008 {published data only}
- Iida H, Iida M, Dohi S, Fukuoka N, Iida M. Preoperative smoking cessation and smoke‐free policy in a university hospital in Japan. Canadian Journal of Anaesthesia 2008;55(5):316‐8. [DOI] [PubMed] [Google Scholar]
Jalilvand 2010 {published data only}
- Jalilvand M, Nikmanesh Z, Kazemi Y, Emamhadi MA. Smokeless tobacco use among university students: A cross‐sectional study in Iran, Sistan Baloochestan Province, 2008. Iranian Journal of Psychiatry and Behavioral Sciences 2010;4:23‐9. [Google Scholar]
Jancey 2014 {published data only}
- Jancey J, Bowser N, Burns S, Crawford G, Portsmouth L, Smith J. No smoking here: Examining reasons for noncompliance with a smoke‐free policy in a large university. Nicotine and Tobacco Research 2014;16:976‐83. [DOI] [PubMed] [Google Scholar]
Jindal 2013 {published data only}
- Jindal AK, Gupta A. Breaking through the smokescreen: A qualitative study of tobacco control in the Indian armed forces. Respiratory Medicine 2013;107:S7. [Google Scholar]
Jovicevic 2009 {published data only}
- Jovicevic Bekic A, Ristic S, Mandic V. Cancer patients' attitudes on smoking, quitting and total ban at cancer hospitals. European Journal of Cancer, Supplement 2009;7 (2‐3):189. [Google Scholar]
Kamath 2011 {published data only}
- Kamath AS, Weg MV, Fu S, Grant K, Prochazka A, Katz D. Nicotine withdrawal and smoking status in hospitalized veterans. Journal of General Internal Medicine 2011;26:S332. [Google Scholar]
Kauffman 2011 {published data only}
- Kauffman RM, Ferketich AK, Murray DM, Bellair PE, Wewers ME. Tobacco use by male prisoners under an indoor smoking ban. Nicotine & Tobacco Research 2011;13:449‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaushik 2013 {published data only}
- Kaushik U, Nazar GP, Yadav A, Arora M, Reddy SK. Protecting from second hand smoke: An assessment of SHS exposure in public places in two states of india. Journal of Thoracic Oncology 2013;8:S998. [Google Scholar]
Kazmi 2010 {published data only}
- Kazmi SMH, Patel S, Watkins L, Tack G, Stead RJ, Babores M. Smoking habits of health care professionals in a district general hospital (DGH) two years after smoking ban. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A26050. [DOI: 10.1164/ajrccm-conference.2010.181.1] [DOI] [Google Scholar]
Kitabayashi 2006 {published data only}
- Kitabayashi Y, Narumoto J, Shibata K, Nakamae T, Kamei J, Sano K, et al. Effect of institutional smoking prohibition on Japanese inpatients with chronic schizophrenia. Nihon Arukoru Yakubutsu Igakkai Zasshi 2006;41(2):128‐33. [PubMed] [Google Scholar]
Lasnier 2011 {published data only}
- Lasnier B, Cantinotti M, Guyon L, Royer A, Brochu S, Chayer L. Implementing an indoor smoking ban in prison: enforcement issues and effects on tobacco use, exposure to second‐hand smoke and health of inmates. Canadian Journal of Public Health. Revue Canadienne de Sante Publique 2011;102(4):249‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laszlo 2013 {published data only}
- Laszlo TD, Domonkos TA, Laszlo C, Travers MJ. Change in secondhand smoke levels in a public hospital in Budapest following anti‐smoking policy implementation in 2011 [Hungarian]. Orvosi Hetilap 2013;154:658‐64. [DOI] [PubMed] [Google Scholar]
Lawn 2014 {published data only}
- Lawn S, Hehir A, Indig D, Prosser S, Macleod S, Keller A. Evaluation of a totally smoke‐free forensic psychiatry in‐patient facility: practice and policy implications. Australian Health Review 2014;38(4):476‐82. [DOI] [PubMed] [Google Scholar]
Lawrence 2008 {published data only}
- Lawrence S, Welfare H. The effects of the introduction of the no‐smoking policy at HMYOI Warren Hill on bullying behaviour. International Journal of Prison Health. 2008;4(3):134‐45. [DOI] [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee JG, Ranney LM, Goldstein AO. Cigarette butts near building entrances: what is the impact of smoke‐free college campus policies?. Tobacco Control 2013;22(2):107‐12. [DOI] [PubMed] [Google Scholar]
Lincoln 2009 {published data only}
- Lincoln T, Tuthill RW, Roberts CA, Kennedy S, Hammett TM, Langmore‐Avila E, et al. Resumption of smoking after release from a tobacco‐free correctional facility. Journal of Correctional Health Care 2009;15(3):190‐6. [DOI] [PubMed] [Google Scholar]
Lotufo 2011 {published data only}
- Lotufo JB, Fernandes F La, Souza ETC, Sampaio PC. Tobacco smoking at BUTANTÃ female prison and FUNDAÇÃO casa unit. American Journal of Respiratory and Critical Care Medicine 2011;183 (1 Meeting Abstracts):A1769. [DOI: ] [Google Scholar]
Lucas 2014 {published data only}
- Lucas A, Calheiros JM, Ravara SB. Prevalence of tobacco use among inpatients in a partial smoke‐free hospital‐ A cross‐sectional study in a Portuguese hospital. European Respiratory Journal 2014;44(58):Abstract. [1399‐3003] [Google Scholar]
Marin 2008 {published data only}
- Marin GH, Silberman M, Ferrero S, Sanguinetti C. Smoking in health institutions in Buenos Aires, Argentina. [Spanish]. Revista del Instituto Nacional de Enfermedades Respiratorias 2008;21:87‐91. [Google Scholar]
Marin 2013 {published data only}
- Marin M, Cliquet S, Pascot E, Kurztag C, Pejoan H, Viriot E. Caregivers and Teenage Smoking in French Hospitals. Turkish Archives of Pediatrics 2013;48:71. [Google Scholar]
Matthews 2005 {published data only}
- Matthews LS, Diaz B, Bird P, Cook A, Stephenson AE, Kraus JE, et al. Implementing a smoking ban in an acute psychiatric admissions unit. Journal of Psychosocial Nursing & Mental Health Services 2005;43(11):33‐6. [DOI] [PubMed] [Google Scholar]
Maurel‐Donnarel 2010 {published data only}
- Maurel‐Donnarel E, Baumstarck‐Barrau K, Barlesi F, Lehucher‐Michel MP. [The ban on smoking in public places (Decree No. 2006‐1386 of 15th November 2006): Impact over 12 months on smoking status of hospital nurses]. Revue des Maladies Respiratoires 2010;27(3):199‐212. [DOI] [PubMed] [Google Scholar]
Mesenge 2014 {published data only}
- Mesenge C, Garanet KFN, Nanema PA, Okoubo G, Keller M, Kevin B. Survey on tobacco addiction at Senghor Uuniversity in Alexandria, Egypt: Mars 2012. Asia‐Pacific Journal of Clinical Oncology 2014;10:126. [Google Scholar]
Michopoulos 2013 {published data only}
- Michopoulos I, Tsaklakidou D, Gournellis R, Rizos E, Christodoulou C, Vasilopoulou K, et al. A 12 month study of smoking prevention in a psychiatric department. European Psychiatry 2013;28(1):Abstract. [Google Scholar]
Mishra 2011 {published data only}
- Mishra S, Thind HK, Gokarakonda SB, Lartey G, Watkins C, Chahal M. Second‐hand smoke in a university campus: Attitudes and perceptions of faculty, staff and students. International Journal of Health Research 2011;4:21‐7. [Google Scholar]
Ohmi 2013 {published data only}
- Ohmi H, Okizaki T, Meadows M, Terayama K, Mochizuki Y. An exploratory analysis of the impact of a university campus smoking ban on staff and student smoking habits in Japan. Tobacco Induced Diseases 2013;11(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pagano 2015 {published data only}
- Pagano A, Guydish J, Le T, Tajima B, Passalacqua E. Change in addiction treatment staff and client smoking following a statewide smoking ban. Drug and Alcohol Dependence 2015;146:e60. [Google Scholar]
Poder 2012 {published data only}
- Poder N, Carroll T, Wallace C, Hua M. Do smoke‐free environment policies reduce smoking on hospital grounds? Evaluation of a smoke‐free health service policy at two Sydney hospitals. Australian Health Review 2012;36(2):158‐62. [DOI] [PubMed] [Google Scholar]
Principe 2013 {published data only}
- Principe R, Paone G, Palermo P, Damante S, Fuselli S, Messano GA, et al. Tobacco smoke: Environmental control in a public hospital of Rome. European Respiratory Journal 2013;42(Suppl 57):1077. [Google Scholar]
Proescholdbell 2008 {published data only}
- Proescholdbell SK, Foley KL, Johnson J, Malek SH. Indoor air quality in prisons before and after implementation of a smoking ban law. Tobacco Control 2008;17(2):123‐7. [DOI] [PubMed] [Google Scholar]
Ratschen 2008 {published data only}
- Ratschen E, Britton J, McNeill A. Smoke‐free hospitals ‐ the English experience: results from a survey, interviews, and site visits. BMC Health Services Research 2008;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ratschen 2009a {published data only}
- Ratschen E, Britton J, Doody GA, McNeill A. Smoke‐free policy in acute mental health wards: avoiding the pitfalls. General Hospital Psychiatry 2009;31(2):131‐6. [DOI] [PubMed] [Google Scholar]
Ratschen 2009b {published data only}
- Ratschen E, Britton J, McNeill A. Implementation of smoke‐free policies in mental health in‐patient settings in England. British Journal of Psychiatry 2009;194(6):547‐51. [DOI] [PubMed] [Google Scholar]
Ritter 2012 {published data only}
- Ritter C, Huynh CK, Etter JF, Elger BS. Exposure to tobacco smoke before and after a partial smoking ban in prison: indoor air quality measures. Tobacco Control 2012;21(5):488‐91. [DOI] [PubMed] [Google Scholar]
Rossi 2012 {published data only}
- Rossi P, Martin Subero M, Sellart Altisent M, Perez De Heredia Flores JL, Delgado Cano V, Torrens Melich M. Implementation of no‐smoking policy: Firstyear experience in a Spanish detoxification unit. European Neuropsychopharmacology 2012;22:S412. [Google Scholar]
Sabidó 2006 {published data only}
- Sabidó M, Sunyer J, Masuet C, Masip J. Hospitalized smokers: compliance with a nonsmoking policy and its predictors. Preventive Medicine 2006;43(2):113‐6. [DOI] [PubMed] [Google Scholar]
See 2014 {published data only}
- See JHJ, Lum YC. Smoke‐free laws and trends in tobacco quit rates: Perspective from a single centre inpatient smoking cessation programme. European Journal of Preventive Cardiology 2014;35(1):P330. [Google Scholar]
Sheffer 2009 {published data only}
- Sheffer C, Stitzer M, Wheeler JG. Smoke‐free medical facility campus legislation: support, resistance, difficulties and cost. International Journal of Environmental Research & Public Health [Electronic Resource] 2009;6(1):246‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shetty 2010 {published data only}
- Shetty A, Alex R, Bloye D. The experience of a smoke‐free policy in a medium secure hospital. Psychiatrist 2010;34:287‐9. [Google Scholar]
Sosman 2010 {published data only}
- Sosman J, Seal D, Kim SY, Thibodeau L, Jorenby D. The effect of a statewide prison smoking ban on smoking behavior after release to the community‐quantitative findings. Journal of General Internal Medicine 2010;25:S400. [Google Scholar]
Stockings 2014 {published data only}
- Stockings EA, Bowman JA, Bartlem KM, McElwaine KM, Baker AL, Terry M, et al. Quality of implementation of a smoke‐free policy in an inpatient psychiatric facility: Association with patient acceptability. Asia‐Pacific Journal of Clinical Oncology 2014;10:166‐7. [Google Scholar]
Sureda 2014 {published data only}
- Sureda X, Ballbè M, Martínez C, Fu M, Carabasa E, Saltó E, et al. Impact of tobacco control policies in hospitals: Evaluation of a national smoke‐free campus ban in Spain. Preventive Medicine Reports 2014;1:56‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tarnoki 2013 {published data only}
- Tarnoki DL, Tarnoki AD, Csathy L, Travers MJ. [Change of secondhand smoke levels in a public hospital in Budapest after implementation of anti‐smoking policy in 2011]. Orvosi Hetilap 2013;154:658‐64. [DOI] [PubMed] [Google Scholar]
Thornley 2013 {published data only}
- Thornley S, Dirks KN, Edwards R, Woodward A, Marshall R. Indoor air pollution levels were halved as a result of a national tobacco ban in a New Zealand prison. Nicotine & Tobacco Research 2013;15:343‐7. [DOI] [PubMed] [Google Scholar]
Tripathy 2013 {published data only}
- Tripathy JP, Goel S, Patro BK. Compliance monitoring of prohibition of smoking (under section‐4 of COTPA) at a tertiary health‐care institution in a smoke‐free city of India. Lung India 2013;30(4):312‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Unrod 2012 {published data only}
- Unrod M, Oliver JA, Heckman BW, Simmons VN, Brandon TH. Outdoor smoking ban at a cancer center: attitudes and smoking behavior among employees and patients. Journal of Public Health Management & Practice 2012;18(5):E24‐31. [DOI] [PubMed] [Google Scholar]
Vardavas 2009 {published data only}
- Vardavas CI, Bouloukaki I, Linardakis MK, Tzilepi P, Tzanakis N, Kafatos AG. Smoke‐free hospitals in Greece: Personnel perceptions, compliance and smoking habit. Tobacco Induced Diseases 2009;5(1):8. [DOI: 10.1186/1617-9625-5-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Voci 2010 {published data only}
- Voci S, Bondy S, Zawertailo L, Walker L, George TP, Selby P. Impact of a smoke‐free policy in a large psychiatric hospital on staff attitudes and patient behavior. General Hospital Psychiatry 2010;32(6):623‐30. [DOI] [PubMed] [Google Scholar]
Vorspan 2009 {published data only}
- Vorspan F, Bloch V, Guillem E, Dupuy G, Pirnay S, Jacob N, et al. Smoking ban in a psychiatry department: Are nonsmoking employees less exposed to environmental tobacco smoke?. European Psychiatry 2009;24(8):529‐32. [DOI] [PubMed] [Google Scholar]
Wheeler 2007 {published data only}
- Wheeler JG, Pulley L, Felix HC, Bursac Z, Siddiqui NJ, Stewart MK, et al. Impact of a smoke‐free hospital campus policy on employee and consumer behavior. Public Health Reports 2007;122(6):744‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wye 2014 {published data only}
- Wye P, Gow LB, Constable J, Bowman J, Lawn S, Wiggers J. Observation of the extent of smoking in a mental health inpatient facility with a smoke‐free policy. BMC Psychiatry 2014;14:94. [DOI: 10.1186/1471-244X-14-94] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xiao 2013 {published data only}
- Stillman FA, Kaufman MR, Zhen A, Yang J, Wang J, Zhao N. Smoke‐free or not: a pilot evaluation in selected Beijing Hospitals. BMC Public Health 2013;13:964. [DOI: 10.1186/1471-2458-13-964] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Wang C, Chen H, Hajek P. Making hospitals in China smoke‐free: a prospective study of implementing the new standard. Nicotine & Tobacco Research 2013;15(12):2076‐80. [DOI] [PubMed] [Google Scholar]
Additional references
Baric 1993
- Baric L. The settings approach‐ implications for policy and strategy. Journal of the Institute of Health Education 1993;31:17‐24. [Google Scholar]
Been 2014
- Been J, Nurmatov U, Cox B, Nawrot T, Schayak C, Sheikh A. Effect of smoke‐free legislation on perinatal and child health: a systematic review and meta analysis. Lancet 2014;383(9928):1549‐60. [DOI] [PubMed] [Google Scholar]
Cahill 2014
- Cahill K, Lancaster T. Workplace interventions for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD003440.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Callinan 2010
- Callinan JE, Clarke A, Doherty K, Kelleher C. Legislative smoking bans for reducing secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD005992.pub2] [DOI] [PubMed] [Google Scholar]
Carson 2011
- Carson KV, Brinn MP, Labiszewski NA, Esterman AJ, Chang AB, Smith BJ. Community interventions for preventing smoking in young people. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD001291.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christie 2014
- Christie B. Smoking ban is upheld at Scotland’s State Hospitaldespite patient’s earlier victory. BMJ 2014;349:g5164. [DOI: 10.1136/bmj.g5164] [DOI] [PubMed] [Google Scholar]
Coppo 2014
- Coppo A, Galanti MR, Giordano L, Buscemi D, Bremberg S, Faggiano F. School policies for preventing smoking among young people. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD009990.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fichtenberg 2002
- Fichtenberg CM, Glantz SA. Effect of smoke‐free workplaces on smoking behaviour: systematic review. BMJ 2002;325(7357):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzpatrick 2009
- Fitzpatrick P, Gilroy I, Doherty K, Corradino D, Daly L, Clarke A, et al. Implementation of a campus‐wide Irish hospital smoking ban in 2009: prevalence and attitudinal trends among staff and patients in lead up. Health Promotion International 2009;24(3):211‐22. [DOI] [PubMed] [Google Scholar]
Frazer 2016
- Frazer K, Callinan JE, McHugh J, Baasel S, Clarke A, Doherty K, et al. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD005992.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ginn 2013
- Ginn S, Robinson R. Promoting health in prison. BMJ 2013;346:f2216. [DOI: 10.1136/bmj.f2216] [DOI] [PubMed] [Google Scholar]
Glasgow 1999
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE‐AIM framework. American Journal of Public Health 1999;89(9):1322‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glasgow 2006
- Glasgow RE, Kleges LM, Dzewaltowski DA, Estabrooks PA, Vogt TM. Evaluting the impact of health promotion programs: using RE‐AIm framework to form summary measures for decision making involving complex issues. Health Education Research 2006;21(5):688‐94. [DOI] [PubMed] [Google Scholar]
GOI 2004
- Government of Ireland. Public Health (Tobacco) (Amendment) Act 2004. www.hse.ie/eng/about/Who/TobaccoControl/legres/publichealth04.pdf (accessed 13th May 2016).
Green 2006
- Green LW, Glasgow RE. Evaluting the relevance, generalization, and applicability of research. Issues in external validation and translation methodology. Evaluation and The Health Professionals 2006;29(1):126‐53. [DOI] [PubMed] [Google Scholar]
Green 2015
- Green J, Tones K, Cross R, Woodall J. Health Promotion. Planning and Strategies. 3. London: Sage Publications Ltd, 2015. [Google Scholar]
Hartwig 2008
- Hartwig C, Stover H, Weilandt C. Report on Tobacco Smoking in Prison. Drug policy and harm reduction SANCO/2006/C4/02 2008.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hrbak‐Zerbajic 2004
- Hrabak‐Zserbajic V. Country Report on Advertising and Promotion Bans‐ Croatia. World Health Organization www.who.int/tobacco/training/success_stories/en/advertising_and_promotion_bans_croatia.pdf 2004 (accessed 13th May 2016).
IARC 2008
- International Agency for Research on Cancer, World Health Organization. IARC Handbooks of Cancer Prevention, Tobacco Control, Vol. 12: Methods for Evaluating Tobacco Control Policies (2008: Lyon, France). www.iarc.fr/en/publications/pdfs‐online/prev/handbook12/ (accessed 13th May 2016).
IARC 2009
- International Agency for Research on Cancer, World Health Organization. IARC Handbooks of Cancer Prevention, Tobacco Control, Vol. 13: Evaluating the effectiveness of smoke‐free policies (2009: Lyon, France). www.iarc.fr/en/publications/pdfs‐online/prev/handbook13/ (accessed 13th May 2016).
Kelleher 2014
- Kelleher C, Frazer K. An international smoking ban: how many lives will be saved. Current Athersclerosis 2014;16(6):418. [DOI] [PubMed] [Google Scholar]
Lawn 2015
- Lawn S, Feng Y, Tsourtos G, Campion J. Mental health professionals' perspectives on the implementation of smoke‐free policies in psychiatric units across England. International Journal of Social Psychiatry 2015;61(5):465‐74. [DOI] [PubMed] [Google Scholar]
Lee 2014
- Lee P, Fry J, Forey B. A review of the evidence on smoking bans and incidence of heart disease. Regulatory Toxicology and Pharmacology 2014;70(1):7‐23. [DOI] [PubMed] [Google Scholar]
MacKay 2016
- MacKay A. The human rights implications of smoking bans in closed environments: What Australia may learn from the international experience. International Journal of Law, Crime and Justice 2015; in press. [DOI: 10.1016/j.ijlcj.2015.12.005] [DOI]
McCaffery 2012
- McCaffery M, Goodman P, Gavigan A, Kenny C, Hogg C, Byrne L, et al. Should any workplace be exempt from smoke‐free law: the Irish experience. Journal of Environmental and Public Health 2012:545483. [DOI: 10.1155/2012/545483] [DOI] [PMC free article] [PubMed]
McManus 2010
- McManus S, Meltzer H, Campion J. Cigarette Smoking and Mental Health in England. Data from the Adult Psychiatric Morbidity Survey 2007. National Centre for Social Research 2010.
Ministry of Justice 2015
- Ministry of Justice. Letter from Prisons Minister Andrew Selous to Robert Neill MP, Chairman of the Justice Select Committee regarding smoking in prisons. https://www.gov.uk/government/speeches/smoking‐in‐prisons 2015.
NICE 2013
- National Institute for Health and Care Excellence. Smoking: acute, maternity and mental health services. www.nice.org.uk/guidance/ph48 2013 (accessed 13th May 2016). [nice.org.uk/guidance/ph48]
O'Dowd 2005
- O'Dowd A. US experience of smokefree prisons. BMJ 2005;331:1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rigotti 2012
- Rigotti NA, Clair C, Munafò MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD001837.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Royal College of Physicians 2013
- Royal College of Physicians, Royal College of Psychiatrists. Smoking and mental health. www.rcpsych.ac.uk/usefulresources/publications/collegereports/cr/cr178.aspx 2013 (accessed 13th May 2016).
Stanton 2013
- Stanton A, Grimshaw G. Tobacco cessation interventions for young people. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD003289.pub5] [DOI] [PubMed] [Google Scholar]
Tan 2012
- Tan CE, Glantz SA. Association between smoke‐free legislation and hospitalizations for cardiac, cerebrovascular, and respiratory diseases: a meta‐analysis. Circulation 2012;126(18):2177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2013
- Thomas RE, McLellan J, Perera R. School‐based programmes for preventing smoking. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD001293.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
US Department of Health and Human Services 2014
- U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. Printed with corrections, January 2014. www.surgeongeneral.gov/library/reports/50‐years‐of‐progress/full‐report.pdf (accessed 13th May 2016).
West 2005
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100(3):299‐303. [DOI] [PubMed] [Google Scholar]
WHO 1978
- World Health Organization. Declaration of Alma‐Ata. International Conference on Primary Health Care, Alma‐Ata, USSR, 6‐12 September 1978. www.who.int/publications/almaata_declaration_en.pdf (accessed 13th May 2016).
WHO 1986
- World Health Organization. The Ottawa Charter for Health Promotion. First International Conference on Health Promotion, Ottawa, 21 November 1986. www.who.int/healthpromotion/conferences/previous/ottawa/en/ (accessed 13th May 2016).
WHO 2002
- World Health Organization. Innovative Care for Chronic Conditions: Building Blocks for Action: Global Report. 2002. www.who.int/chp/knowledge/publications/icccreport/en/ (accessed 13th May 2016).
WHO 2003
- World Health Organization. WHO Framework Convention on Tobacco Control. 2003. www.who.int/tobacco/framework/WHO_FCTC_english.pdf (accessed 13th May 2016).
WHO 2008
- World Health Organization. WHO Report on the Global Tobacco Epidemic, 2008 ‐ The MPOWER package. www.who.int/tobacco/mpower/2008/en/ (accessed 13th May 2016).
WHO 2009
- World Health Organization. WHO Report on the Global Tobacco Epidemic, 2009: implementing smoke‐free environments. www.who.int/tobacco/mpower/2009/en/ (accessed 13th May 2016).
WHO 2013a
- World Health Organization. WHO report on the global tobacco epidemic 2013: enforcing bans on tobacco advertising, promotion and sponsorship. www.who.int/tobacco/global_report/2013/en/ (accessed 13th May 2016).
WHO 2013b
- World Health Organization. The Helsinki Statement on Health in All Policies. The 8th Global Conference on Health Promotion, Helsinki, Finland, 10‐14 June 2013. www.who.int/healthpromotion/conferences/8gchp/8gchp_helsinki_statement.pdf (accesseed 13th May 2016).
WHO 2014
- World Health Organization. 2014 global progress report on implementation of the WHO Framework Convention on Tobacco Control. www.who.int/fctc/reporting/2014globalprogressreport.pdf (accessed 13th May 2016).
References to other published versions of this review
Frazer 2015
- Frazer K McHugh J Callinan JE Kelleher C. Impact of institutional smoking bans on reducing harms and secondhand smoke exposure . Cochrane Database of Systematic Reviews 2015 , Issue Issue 9 . [CENTRAL: CD011856; DOI: 10.1002/14651858.CD011856] [DOI] [PMC free article] [PubMed] [Google Scholar]


