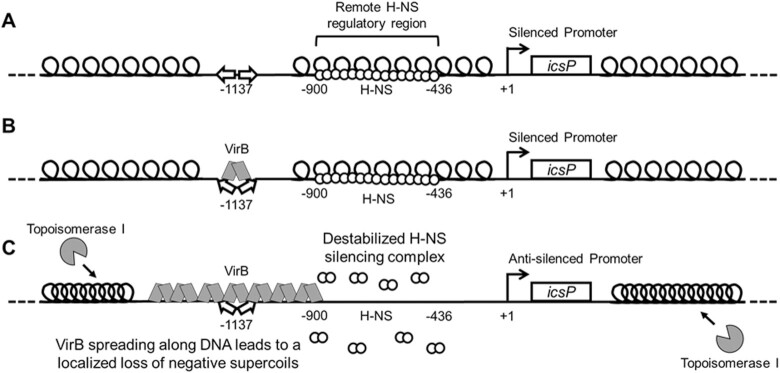
Figure 8.
Model showing the proposed mechanism of VirB-induced supercoiling and its alleviation of H-NS-mediated transcriptional silencing. (A) At ambient temperatures, 30°C, the PicsP-lacZ reporter is negatively supercoiled and transcriptionally silenced by H-NS, which binds to a remote region located between -900 to –436 relative to the primary icsP TSS (49). (B) Upon a switch to 37°C, VirB docks to its binding site as a dimer (49–50,61), inducing a slight bend in the DNA (60). (C) VirB subsequently oligomerizes along DNA bi-directionally (49,61,73). VirB spreading along the DNA toward H-NS is required for transcriptional anti-silencing (49), leading to a loss of negative DNA supercoils in the region bound by H-NS. This loss of negative supercoils destabilizes or remodels the H-NS-silencing complex, rendering the promoter permissive for transcription. The topological changes mediated by VirB lead to the accrual of negative supercoils in unoccupied regions of the plasmid, which attract topoisomerase I to restore negative supercoiling to normal levels, which allowed VirB-mediated changes in supercoiling to be captured in our assays. Thus, the anti-silencing activity of VirB is mediated directly through VirB:DNA interactions, topoisomerase I may contribute, but is not the primary driver of this regulatory process.