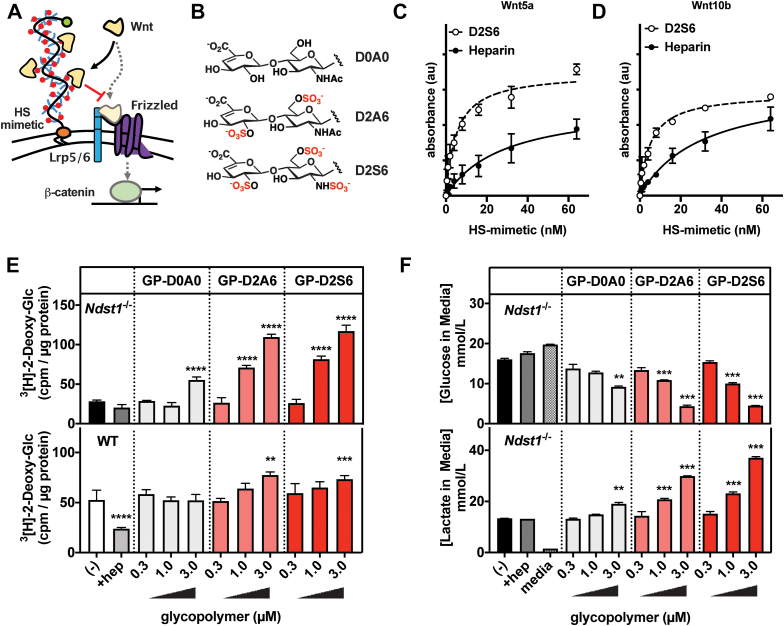
Figure 6.
Membrane-incorporation of synthetic HS glycopolymers during adipogenesis rescues the impaired glucose uptake in HS-deficient adipocytes.A, synthetic glycopolymers bearing a HS GAG disaccharide repeats were prepared and incorporated into the membranes of differentiating MEFs. Schematic of Wnt ligand sequestration by cell membrane anchored HS glycopolymers. B, selected HS GAG disaccharides D2A6 (disulfated), D2S6 (trisulfated), and D0A0 (unsulfated). C, Wnt5a binding activity assessed by ELISA with D2S6 glycopolymer (EC50 = 5.1 nM, r2 = 0.94, n = 3) or heparin (EC50 = 28.2 nM, r2 = 0.87, n = 3), showing dose-dependent binding activity. D, Wnt10b binding activity assessed by ELISA with D2S6 glycopolymer (EC50 = 5.1 nM, r2 = 0.97, n = 2) or heparin (EC50 = 30.1 nM, r2 = 0.95, n = 3), showing dose-dependent binding activity. E, Ndst1−/− and WT MEFs are treated with glycopolymer for 1 h at 37 °C on day 0 to 3 of adipocyte differentiation. Sulfated glycopolymers D2A6 and D2S6 are able to dose-dependently enhance 3[H]-2-deoxy-glucose uptake in differentiated adipocytes (day 6) (two-way ANOVA, n = 3). F, media of treated Ndst1−/− adipocytes (day 6) was assessed for glucose and lactate concentration using YSI. Cells treated with sulfated glycopolymers show a dose–response rescue of glucose utilization (lowered media concentration) and increased production of the glycolysis product lactate (two-way ANOVA, n = 2). Data are presented as mean ± SD, ∗∗∗∗p < 0.0001, ∗∗∗p < 0.001, ∗∗p < 0.01. HS, heparan sulfate; MEF, mouse embryonic fibroblast; Ndst1, N-deacetylase-N-sulfotransferase 1.