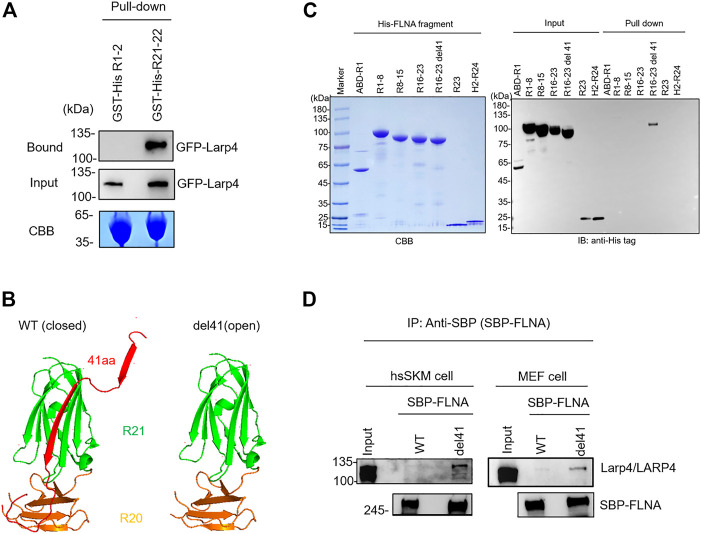
FIGURE 2.
Selective interaction of LARP4 with open FLNA. (A) GFP-Larp4 was expressed in HEK 293A cells and the expressed protein was pulled down with purified GST-His-FLNA fragments. CBB staining showed 5 μg of the purified GST fusion protein loaded to the 9% SDS-PAGE gel. Bound GFP-Larp4 was detected by western blotting using rabbit anti-GFP antibodies. (B) The CD face of R21 is blocked with strand A of R20 in WT FLNA (left). Deletion of 41 amino acid residues (del41) constitutively exposes the cryptic binding site (right). (C) Mapping of Larp4-binding site on FLNA. Purified His-tagged FLNA fragments were pulled down with GST-Larp4 immobilized on glutathione beads. CBB staining showed 2 μg of the purified His-tagged FLNA fragments protein loaded to the 4%–12% SDS-PAGE gel. The lower bands of the input proteins are indicative of degradation during expression in bacteria. Black arrow indicates the GST-Larp4. Bound His-tag FLNA fragments were detected by western blotting using anti-His-tag antibody. (D) SBP-FLNA (WT and del41) was expressed in HEK293A cells and immobilized on streptavidin-beads. The beads were incubated with lysates of hsSKM (left) or MEF (right) cell and bound Larp4/LARP4 was detected by western blotting using anti-LARP4 antibodies. CBB: Coomassie Brilliant Blue. IB: Immunoblot. IP: Immunoprecipitation.