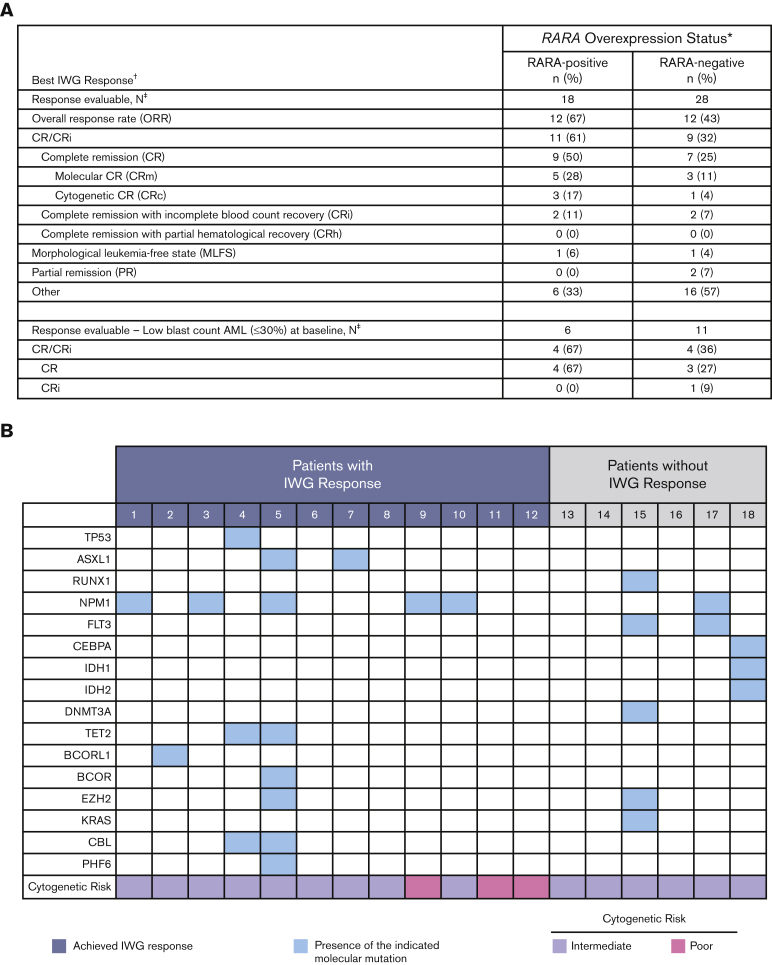
Figure 3.
Summary of OR. (A) Summary of best OR in ND unfit patients with AML. Table shows a summary of the best efficacy response achieved by all response-evaluable patients. The response-evaluable population comprised all patients enrolled who (1) completed 1 cycle of tamibarotene and had a follow-up assessment of disease status or (2) were withdrawn from the study before completion of cycle 1 because of documented disease progression. Patients listed in the “other” category did not achieve an International Working Group (IWG) response. ∗RARA overexpression was determined in blasts isolated from PBMCs by qRT-PCR assay. The presence of RARA overexpression was characterized as RARA-positive, and the absence of RARA overexpression as RARA-negative; †Disease status was assessed per the revised IWG AML criteria;13,14 ‡All response-evaluable patients. (B) Association of IWG response with DNA mutations and cytogenetic risk in RARA-positive patients. Data are shown for the 18 RARA-positive response evaluable patients. Cytogenetic risk was assessed per National Comprehensive Cancer Network (NCCN) AML guidelines 2018.15 The mutation profiles and cytogenetic risk of patients were site reported. Response was assessed per the revised IWG AML criteria.13,14