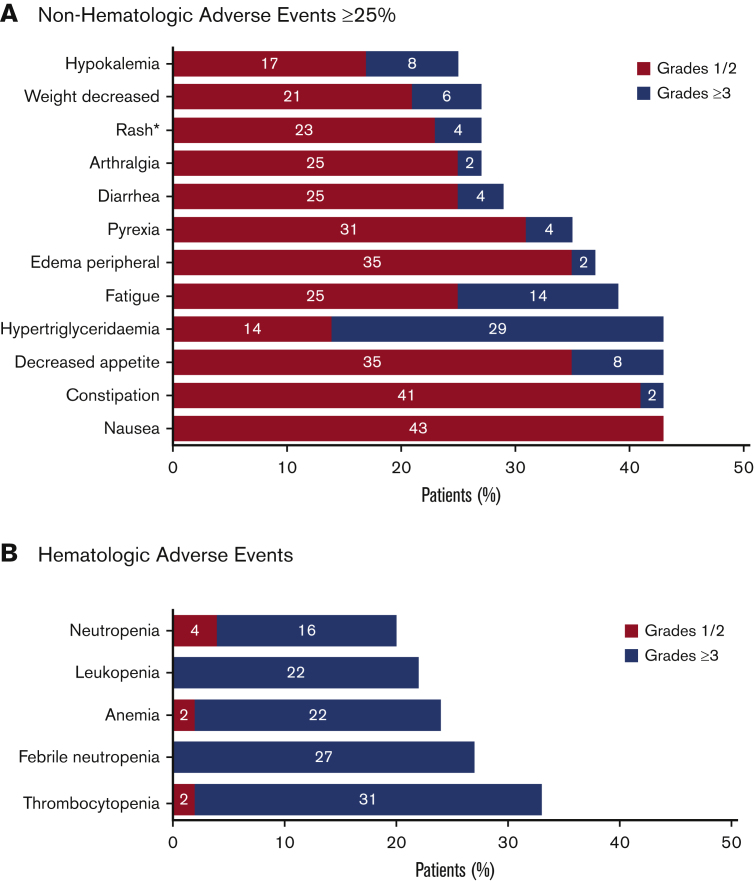
Figure 5.
Summary of AEs. All treatment emergent AEs for all enrolled patients (N = 51) were evaluated. The safety population included all patients who received at least 1 dose of study drug (tamibarotene or azacitidine). AEs were evaluated using Common Terminology Criteria for Adverse Events version 4.03. (A) Nonhematologic AEs that were reported in at least 25% of patients. *The term “rash” included the preferred terms of rash maculo-papular, rash, drug eruption, nodular rash, rash erythematous, and rash pruritic. Rash maculopapular and rash were each reported in 5 (10%) of patients, with other terms reported in 1 patient each (2%). (B) Hematologic AEs.