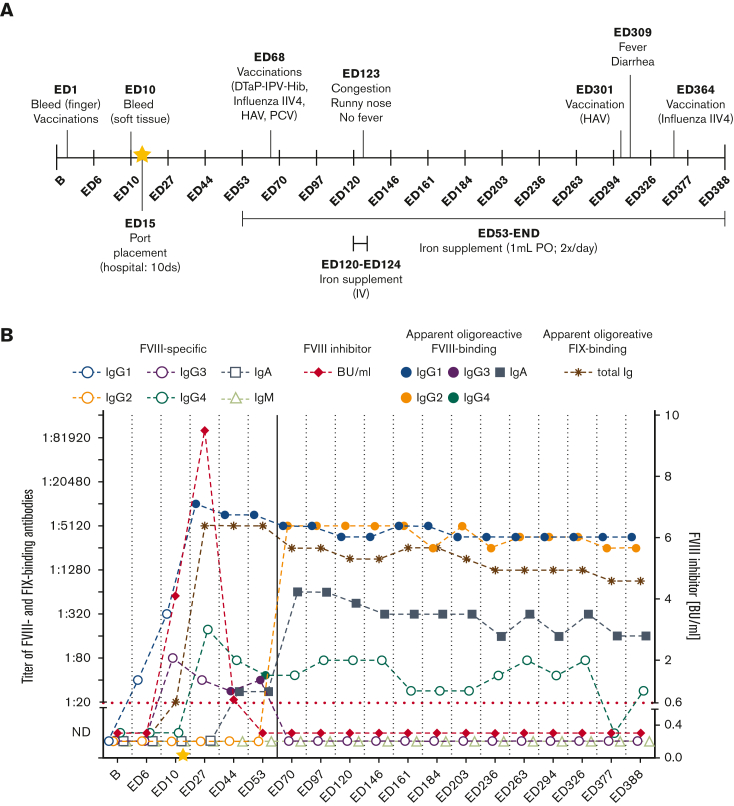
Figure 5.
Apparent oligoreactive FVIII- and FIX-binding antibodies in HIPS-ITI patient 2 during HIPS and HIPS-ITI and timeline of clinical events. (A) Timeline of clinical events documented for HIPS-ITI patient 2 throughout HIPS and HIPS-ITI including (i) adverse events with short descriptions, (ii) infections and administered treatment, (iii) hospitalizations, duration of hospitalizations and administered treatment, (iv) immunizations and administered vaccinations, and (v) additionally administered medications and supplements. The yellow star marks the initiation of ITI treatment at ED16. (B) Apparent oligoreactive FVIII- (points, filled squares) and FIX-binding (asterisks) antibody titers as well as FVIII-specific (circles, open squares, open triangles) antibody titers (total Ig, IgG1, IgG2, IgG3, IgG4, IgA, IgM as indicated) and FVIII inhibitors (BU/mL) for HIPS-ITI patient 2. The red dotted line represents the limit for positive evaluation of FVIII inhibitors (0.6 BU/mL). The yellow star marks the initiation of ITI treatment at ED16. The continuous vertical line indicates the end of HIPS and the start of HIPS-ITI. DTaP-IPV-Hib, diphtheria, tetanus, pertussis, inactivated polio vaccine, Haemophilus influenzae type b; IIV4, quadrivalent-inactivated influenza vaccine; HAV, hepatitis A virus; PCV, pneumococcal-conjugated vaccine; PO, by mouth.