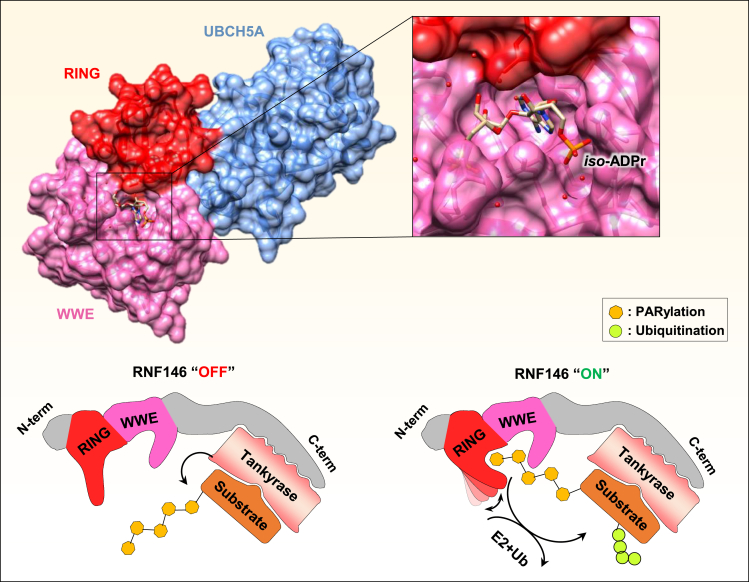
Figure 7.
The mechanism of PARylation-triggered ubiquitination
(Top) The crystal structure of RNF146 WWE/RING domains associated with the UBCH5A E2 conjugating enzyme shows a binding pocket for iso-ADPr within the WWE domain and with additional contact with the RING domain (PDB: 4QPL). (Bottom) PARylation of a target protein by the Tankyrase interacting with RNF146. This binding triggers an allosteric conformational change within the RING domain of RNF146 switching its E3 ligase activity from an “OFF” to an “ON” state leading to efficient recruitment of E2 enzyme and ubiquitination of the PARylated target proteins.