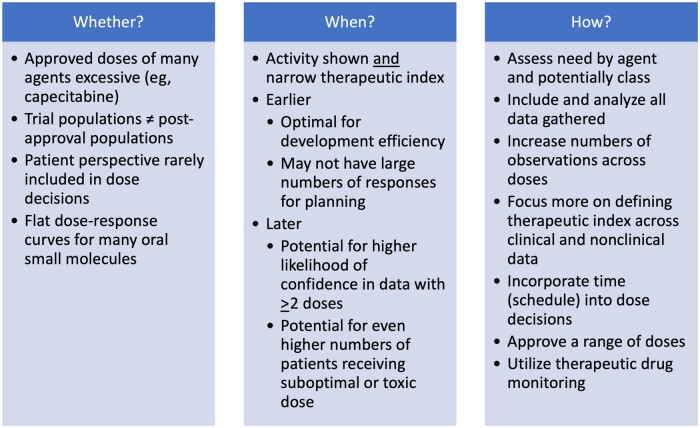
In this issue of the Journal, Korn et al. (1) posit dose optimization during anticancer agent development should occur only after the investigational agent has shown clinical activity and that “patient and public health interests may be better served” if performed after phase II or III testing vs in first-in-human or other early-phase assessments. The title provides a convenient launching point because “whether and when?” are important questions, but for many, including patients, investigators, and the pharmaceutical industry, the most pressing question has been “how?” (Figure 1).
Figure 1.
Considerations in changing the dose derivation paradigm.
Whether?
Conversations around improving dose optimization in oncology have been building over time. One could make the case that the poster child for this discussion is capecitabine, whose original labeled dose in 1998 was 1250 mg/m2 orally twice daily and had the caveat “dosage may need to be individualized to optimize patient management” and was rarely given at the labeled dose in subsequent trials and in clinics. The Food and Drug Administration, through Project Renewal, recognized the need for a change and recently updated the capecitabine label to include lower doses based on high-quality evidence, removing the vague dose language guidance (2). In a review of 59 new oral molecular entities approved 2010-2020, the median dose reduction, interruption, and discontinuation rates in registration trials were 28%, 55%, and 10%, respectively, and did not vary by year of approval, indication (solid tumor vs hematologic malignancy) or molecular target (3). Because these data are from trial volunteers, dose adjustment rates in clinical use are expected to be higher, leading to patients experiencing potentially unnecessary adverse events and lower adherence, clinicians using lower than labeled doses at therapy initiation, and additional uncertainty about the right dose for all patients. Indeed, when surveyed, 65% of generalist oncologists treating patients with metastatic disease agreed that initiating lower than labeled doses to reduce adverse events, even at the expense of efficacy, was justified (4). One must wonder how much of this experience-based belief is driven by historically excessive doses derived from phase I trials and carried forward to registration and approval without deeper investigation of dose range activity and tolerability.
Korn et al. (1) begin with the assumption and fundamental agreement with the Food and Drug Administration and Project Optimus that dose optimization processes should be revised during development, affirming the “whether?” and focus on the substantive concern of “when?”
When?
When to optimize dose in a drug’s development life cycle is a complex question and should therefore have multiple answers rather than a standard approach for all agents regardless of class (oral small molecule, biologic, antibody-drug conjugate, etc), indication(s), and population to be treated. Korn et al. (1) correctly point out that selecting multiple doses for evaluation is feasible only when clinical activity has been demonstrated, “because there are rarely biomarkers available during early drug development that are reproducible and reliable surrogates of clinical benefit.” The authors also express concern that, should randomized trials of dose occur too early in development, an excessive number of patients may be treated with ineffective doses and potentially have inferior cancer outcomes. However, we must also consider the current and historical number of patients treated with excessively toxic doses of agents that have a flattened dose-response relationship. Korn et al. (1) focus on activity and concerns around loss of activity with lower doses and place little emphasis on toxicity in their commentary. Examples of overly toxic doses in practice are many and are predominantly small molecule oral agents developed over the last 1-2 decades. Additionally, current dose selection strategies from phase I trials often do not use all the available data. An approach advocated by Postel-Vinay et al. (5) incorporates a weighting approach to new or worsening adverse events seen beyond cycle 1 when considering dose and next steps in a drug’s development. The adverse events that persist and are more subjective (eg, diarrhea, nausea, fatigue) cause patients to discontinue medications, knowingly or unknowingly to providers. A recent group expanded on this concept and published recommendations for phase I trial designs in the context of Project Optimus, encouraging more flexible designs, real-world eligibility criteria, intrapatient dose escalation, randomization to backfill dose levels, real-time pharmacokinetic and pharmacodynamic data, and use of all available data to select a dose range for further study (6). If these recommendations were adopted and successful, the argument of “when” dose should be optimized would favor that period immediately after the first-in-human experience.
Korn et al. (1) state that “dose optimization should occur after the treatment has shown activity in phase 2.” However, with modern drug development and seamless trials, go or no-go development decisions are made long before initiating phase II trials based on preclinical data and smaller numbers of responses. As one waits longer in the development process, the weight of activity leans greater and greater toward a higher dose; without pursuing knowledge of lower doses earlier, the ethics of reducing dose later become more challenging. Korn et al. (1) also state that early dose optimization as a strategy may increase “the chances of selecting an ineffective dose and treating many patients with ineffective therapy.” I would challenge them to provide an example, particularly of a small molecule, where lower doses identified following marketing have been less effective than a higher dose. If we allow toxic doses that provide no greater efficacy to be approved, we are doing a major disservice to patients. Postmarketing requirements of trials assessing labelled vs lower dose(s) are indicative of a failure of the oncology drug development paradigm.
How?
As noted by Shah et al. (7), other disease areas approach this differently, employing randomized trials of dose following initial dose-ranging studies. However, this comparison fails to fully capture the complexity of drug development in oncology because dose-ranging studies in cancer are performed with agents that may have less robust data and models for on target, mechanistic activity; cancer is a more heterogeneous group of diseases; trials are performed in patients where adverse events may be clouded due to cancer and comorbidities; and greater pressure to develop agents rapidly prevails. Despite these shortcomings, we can learn from other disease approaches. Fundamentally, we must derive therapeutic windows for new agents as early as possible in development and/or have more confidence in surrogate markers for activity. Those agents with traditionally more optimal pharmacodynamic surrogates such as monoclonal antibodies (eg, CD binding assays) have paradoxically had fewer concerns with demonstrating clinical activity early in development as well as fewer roadblocks with dose optimization due to predictable pharmacokinetics and generally more favorable adverse event profiles.
The concept and deployment of agent- and class-specific approaches to dose optimization can and should occur. A one-size-fits-all approach is neither progressive nor tenable; agents that show early activity across dose levels with little to no tolerability concerns may not need to have doses optimized at all. Although this is the current minority of drugs in development, it should be the aspiration. Choices of dose are based on highest likelihood and frequency of response and ideally supported by clinical pharmacology measures. If an agent has shown little to no activity in early-phase trials but has a clear dose-related adverse event profile, the first question must be whether to continue development. If yes, multiple expansion cohorts of dose could be employed in phase I to gain greater numbers of observations for activity assessment. Although this approach may infuriate most statisticians, an investment in a separate fully powered trial to assess preferred dose may not only be futile due to sample sizes needed but will be a waste of precious resources and time.
Another overlooked area is that of schedule and dose density. Korn et al. (1) state “Randomized comparisons of different drug schedules with the same effective dose level have received less attention because scheduling can usually be guided by the pharmacokinetics of the agent.” This is rarely true because schedule is primarily directed by tolerability or adverse events and convenience. Pulsatile schedules followed by rest periods are driven by tolerability, and, compared with other disease areas, oncology is notorious for having complicated oral agent regimens that make adherence for the most vulnerable patients (older, multiple medications, and comorbidities) challenging. Shah et al. (7) state “It’s not unusual for doses and schedules of oncology drugs to be inadequately characterized before sponsors initiate registration trials.” Assessment of multiple schedules, ramped-up dosing approaches, and early, consistent supportive care in phase I trials can assist in improving short- and long-term tolerability and potentially activity.
Should these recommendations and approaches fail to yield the desired outcome, that is, a tolerable effective dose for a population, a final tactic to individualize dose is therapeutic drug monitoring. Groups have long advocated for and investigated target plasma concentrations to improve disease outcomes and tolerability (8,9). I see this as an “and” rather than an “or” for dose optimization in oncology. In a perfect world, agents would be approved with doses normalized to exposures and outcomes across a range based on relevant patient-specific characteristics such as frailty, organ function, concomitant medications, and other factors.
Our goal as trialists should be to ensure all patients are treated safely and with maximal therapeutic intent. The time for dose optimization has arrived, and it cannot happen too soon.
Data availability
No new data were generated or analyzed for this editorial.
Author contributions
R Donald Harvey, PharmD (Writing – original draft; Writing – review & editing)
Funding
No funding was used for this editorial.
Conflicts of interest
RDH: research funding to institution that supports salary: Abbisko, AbbVie, Actuate, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Genmab, GlaxoSmithKline, Infinity, InhibRx, Janssen, Merck, Mersana, Meryx, Morphosys, Novartis, Pfizer, Regeneron, Sanofi, Sutro, Takeda, Turning Point Therapeutics, Xencor. Consultant: Amgen, GlaxoSmithKline.
References
- 1. Korn EL, Moscow JA, Freidlin B.. Dose optimization during drug development: whether and when to optimize. J Natl Cancer Inst. 2023;115(5):492-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capecitabine prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020896s044s051lbl.pdf. Revised December 2022. Accessed January 7, 2023.
- 3. McCabe C, Bryson E, Harvey RD.. Dose derivation of oral anticancer agents: tolerability in late phase registration trials. Eur J Cancer. 2020;138(suppl 2):S51. https://event.eortc.org/ena2020/wp-content/uploads/sites/17/2020/10/EJC-138S2-ENA-2020-abstracts.pdf. Accessed January 7, 2023. [Google Scholar]
- 4. Jimenez RB, Schenkel C, Levit LA, Hu B, et al. Oncologists’ perspectives on individualizing dose selection for patients with metastatic cancer. J Clin Oncol Oncol Pract. 2022;18(11):e1807-e1817. doi: 10.1200/OP.22.00427. [DOI] [PubMed] [Google Scholar]
- 5. Postel-Vinay S, Gomez-Roca C, Molife LR, et al. Phase I trials of molecularly targeted agents: should we pay more attention to late toxicities? J Clin Oncol. 2011;29(13):1728-1735. [DOI] [PubMed] [Google Scholar]
- 6. Araujo D, Greystoke A, Bates S, et al. Oncology phase I trial design and conduct: time for a change – MDICT Guidelines 2022. Ann Oncol. 2023;34(1):48-60. [DOI] [PubMed] [Google Scholar]
- 7. Shah M, Rahman A, Theoret MR, Pazdur R.. The drug-dosing conundrum in oncology – when less is more. N Engl J Med. 2021;385(16):1445-1447. [DOI] [PubMed] [Google Scholar]
- 8. Beumer JH, Chu E, Salamone SJ.. All optimal dosing roads lead to therapeutic drug monitoring – why take the slow lane? JAMA Oncol. 2022;8(12):1733-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Groenland SL, van Eerden RAG, Westerdijk K, et al. Therapeutic drug monitoring-based precision dosing of oral targeted therapies in oncology: a prospective multicenter study. Ann Oncol. 2022;33(10):1071-1082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed for this editorial.