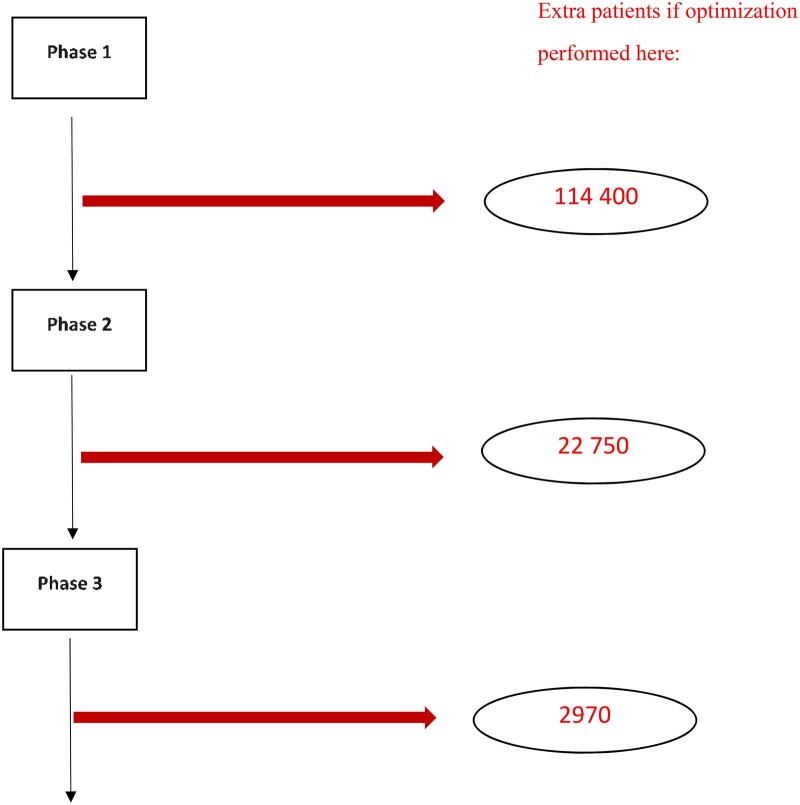
Figure 3.
Based on starting 1000 phase I trials, estimated extra numbers of patients treated with an agent that does not receive FDA approval when 200-patient dose optimization studies are performed at different points in the drug development process. Phase success rates are 63.9%, 28.3%, and 45.2%, and eventual FDA approval rates are 6.7%, 10.5%, and 37.0% for phase I, II, and III trials, respectively [see Table 5 published in reference (35).]