Figure 4.
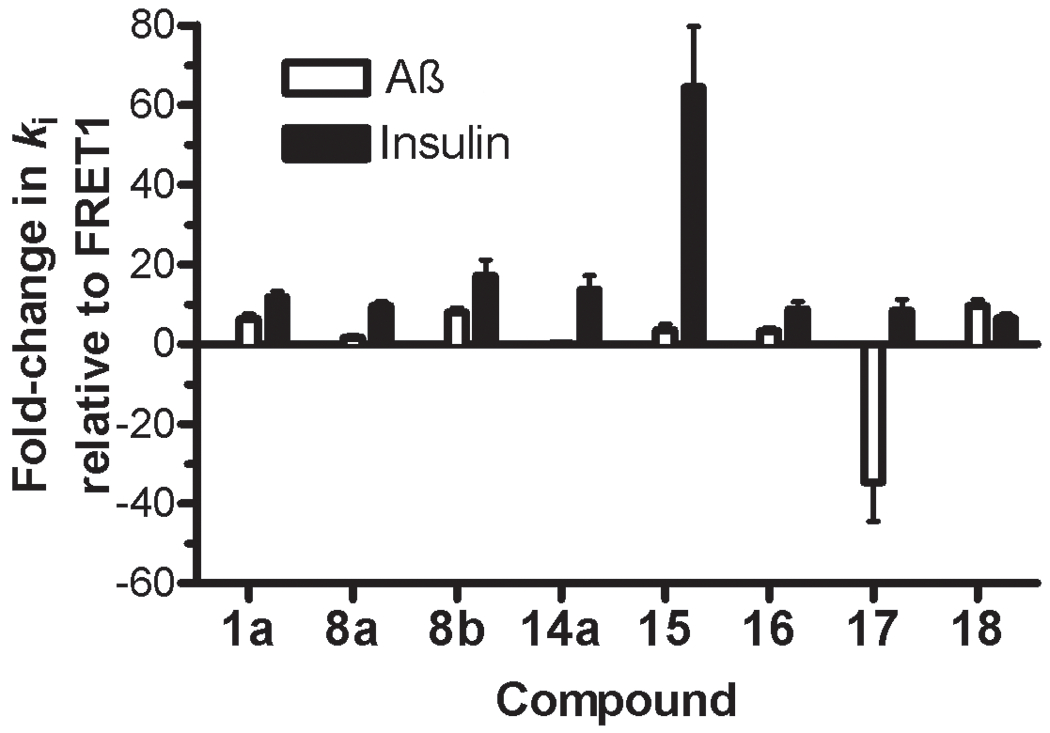
The potency of individual IDE inhibitors is markedly substrate selective. Fold-change in apparent ki (kiapp) values for Aß degradation (open bars) and insulin degradation (solid bars) relative to those obtained with the FRET1 activity assay (derived from Table 5) obtained using inhibitor 1a and the most potent inhibitors developed in this study. Note that most inhibitors show at least 10-fold higher kiapp values for both Aß and insulin degradation relative to FRET1 degradation, an effect that nonetheless varies between different inhibitors. In particular, relative to FRET1 degradation, inhibitor 15 inhibits insulin degradation >60-fold, while inhibitor 17 inhibits the Aß degradation >30-fold more effectively.
