Abstract
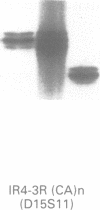
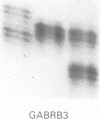
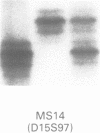
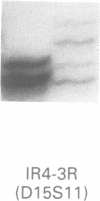
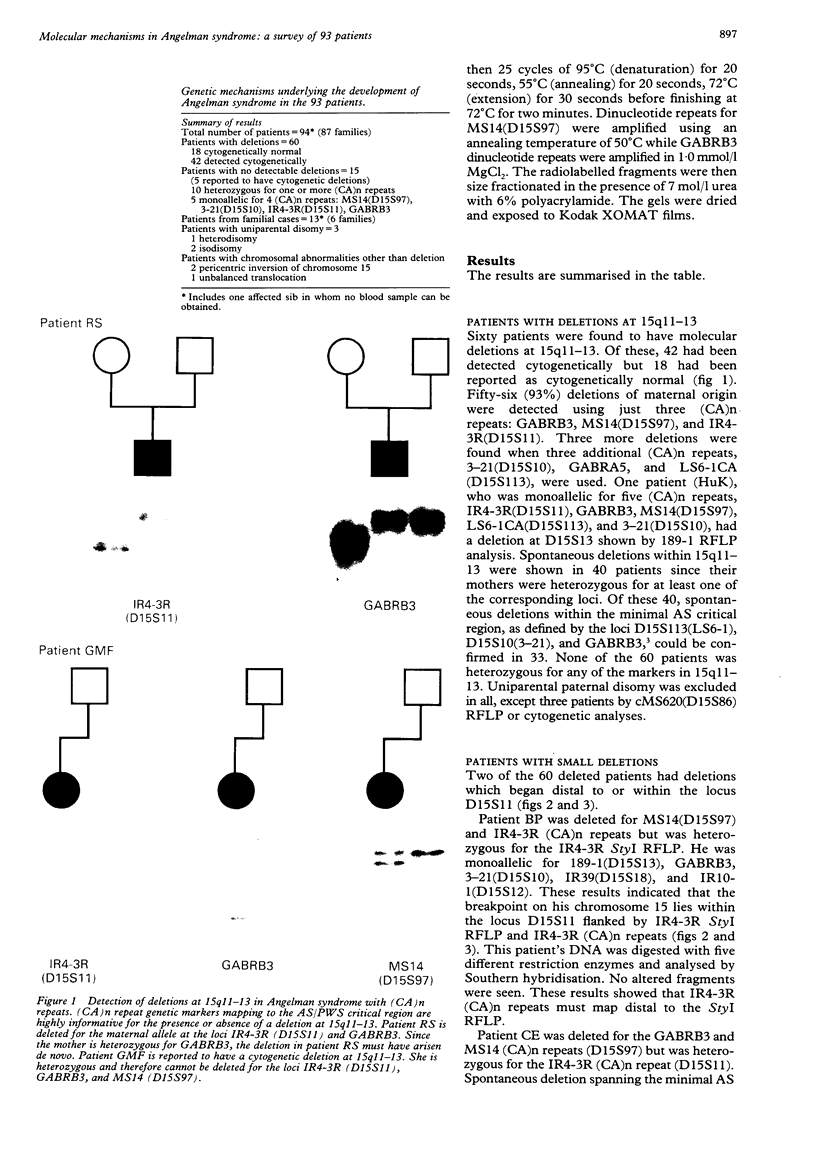
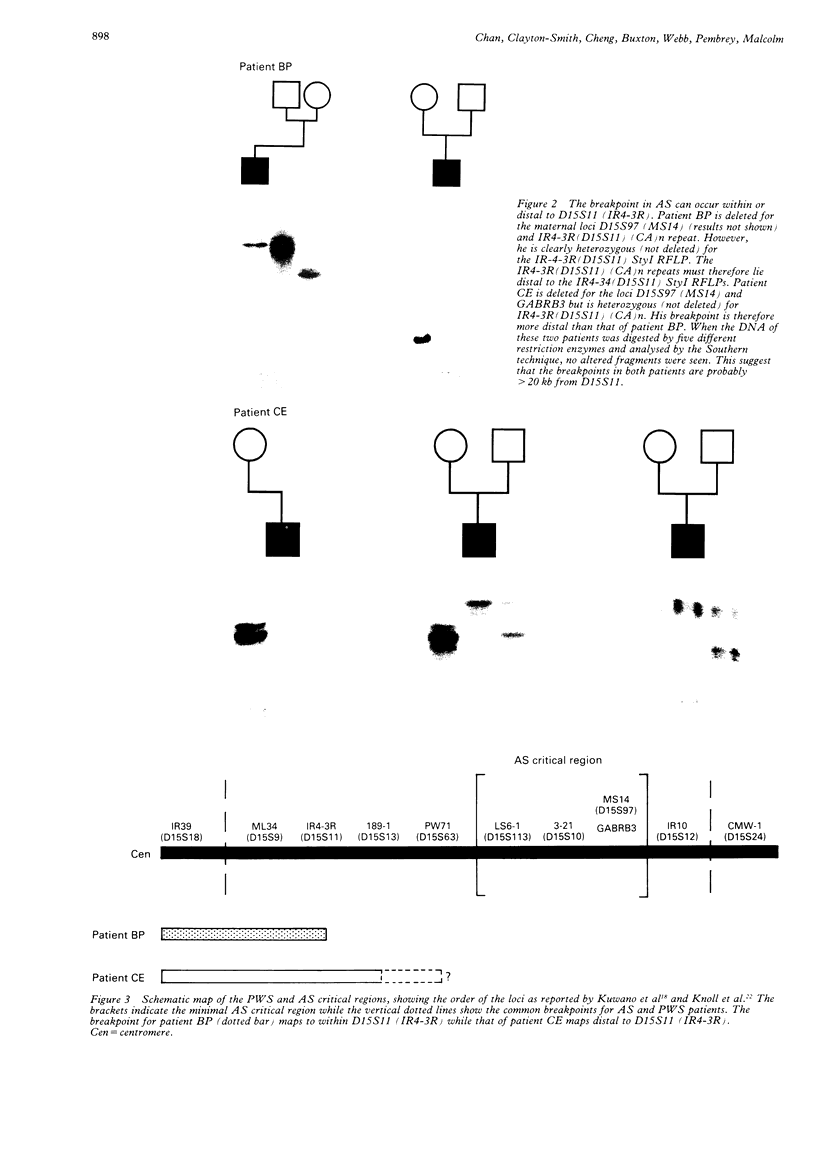
Angelman syndrome (AS) results from a lack of maternal contribution from chromosome 15q11-13, arising from de novo deletion in most cases or rarely from uniparental disomy. These families are associated with a low recurrence risk. However, in a minority of families, more than one child is affected. No deletion has been found in these families, except one. The mode of inheritance in these families is autosomal dominant modified by imprinting. Sporadic cases, with no observable deletion, therefore pose a counselling dilemma as there could be a recurrence risk as high as 50%. We present a series of 93 AS patients, showing the relative contribution of these different genetic mechanisms. Eighty-one AS patients were sporadic cases while 12 cases came from six families. Sixty cases had deletions in 15q11-13 detected by a set of highly polymorphic (CA)n repeats markers and conventional RFLPs. Ten sporadic cases plus all 12 familial cases had no detectable deletion. In addition, two cases of de novo deletions occurred in a chromosome 15 carrying a pericentric inversion. In one of these the AS child had a cousin with Prader-Willi syndrome (PWS) arising from a de novo deletion in an inv(15) inherited from his father. One case arose from a maternal balanced t(9;15)(p24;q15) translocation. There were three cases of uniparental disomy. Five patients were monoallelic for all loci across the minimal AS critical region, but the presence of a deletion cannot be confirmed. In familial cases, all affected sibs inherited the same maternal chromosome 15 markers for the region 15q11-13. Two cases were observed with a de novo deletion starting close to the locus D15S11 (IR4-2R), providing evidence for the development of classical AS with smaller deletions. Cytogenetic analysis proved limited in its ability to detect deletions, detecting only 42 out of 60 cases. However, cytogenetic analysis is still essential to detect chromosomal abnormalities other than deletions such as inversions and balanced translocations since both have an increased risk for deletions. A staged diagnostic strategy based on the use of highly informative (CA)n repeat markers is proposed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armour J. A., Povey S., Jeremiah S., Jeffreys A. J. Systematic cloning of human minisatellites from ordered array charomid libraries. Genomics. 1990 Nov;8(3):501–512. doi: 10.1016/0888-7543(90)90037-u. [DOI] [PubMed] [Google Scholar]
- Beuten J., Mangelschots K., Buntinx I., Coucke P., Brouwer O. F., Hennekam R. C., Van Broeckhoven C., Willems P. J. Molecular study of chromosome 15 in 22 patients with Angelman syndrome. Hum Genet. 1993 Jan;90(5):489–495. doi: 10.1007/BF00217446. [DOI] [PubMed] [Google Scholar]
- Cattanach B. M., Barr J. A., Evans E. P., Burtenshaw M., Beechey C. V., Leff S. E., Brannan C. I., Copeland N. G., Jenkins N. A., Jones J. A candidate mouse model for Prader-Willi syndrome which shows an absence of Snrpn expression. Nat Genet. 1992 Dec;2(4):270–274. doi: 10.1038/ng1292-270. [DOI] [PubMed] [Google Scholar]
- Clayton-Smith J., Pembrey M. E. Angelman syndrome. J Med Genet. 1992 Jun;29(6):412–415. doi: 10.1136/jmg.29.6.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton-Smith J., Webb T., Robb S. A., Dijkstra I., Willems P., Lam S., Cheng X. J., Pembrey M. E., Malcolm S. Further evidence for dominant inheritance at the chromosome 15q11-13 locus in familial Angelman syndrome. Am J Med Genet. 1992 Sep 15;44(2):256–260. doi: 10.1002/ajmg.1320440236. [DOI] [PubMed] [Google Scholar]
- Dittrich B., Robinson W. P., Knoblauch H., Buiting K., Schmidt K., Gillessen-Kaesbach G., Horsthemke B. Molecular diagnosis of the Prader-Willi and Angelman syndromes by detection of parent-of-origin specific DNA methylation in 15q11-13. Hum Genet. 1992 Nov;90(3):313–315. doi: 10.1007/BF00220089. [DOI] [PubMed] [Google Scholar]
- Donlon T. A. Report of the first international workshop on human chromosome 15 mapping. Cytogenet Cell Genet. 1992;61(3):162–166. [PubMed] [Google Scholar]
- Driscoll D. J., Waters M. F., Williams C. A., Zori R. T., Glenn C. C., Avidano K. M., Nicholls R. D. A DNA methylation imprint, determined by the sex of the parent, distinguishes the Angelman and Prader-Willi syndromes. Genomics. 1992 Aug;13(4):917–924. doi: 10.1016/0888-7543(92)90001-9. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Glatt K. A., Sinnett D., Lalande M. Dinucleotide repeat polymorphism at the GABAA receptor alpha 5 (GABRA5) locus at chromosome 15q11-q13. Hum Mol Genet. 1992 Aug;1(5):348–348. doi: 10.1093/hmg/1.5.348. [DOI] [PubMed] [Google Scholar]
- Hamabe J., Kuroki Y., Imaizumi K., Sugimoto T., Fukushima Y., Yamaguchi A., Izumikawa Y., Niikawa N. DNA deletion and its parental origin in Angelman syndrome patients. Am J Med Genet. 1991 Oct 1;41(1):64–68. doi: 10.1002/ajmg.1320410117. [DOI] [PubMed] [Google Scholar]
- Hultén M., Armstrong S., Challinor P., Gould C., Hardy G., Leedham P., Lee T., McKeown C. Genomic imprinting in an Angelman and Prader-Willi translocation family. Lancet. 1991 Sep 7;338(8767):638–639. doi: 10.1016/0140-6736(91)90652-6. [DOI] [PubMed] [Google Scholar]
- Jeanpierre M. A rapid method for the purification of DNA from blood. Nucleic Acids Res. 1987 Nov 25;15(22):9611–9611. doi: 10.1093/nar/15.22.9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll J. H., Glatt K. A., Nicholls R. D., Malcolm S., Lalande M. Chromosome 15 uniparental disomy is not frequent in Angelman syndrome. Am J Hum Genet. 1991 Jan;48(1):16–21. [PMC free article] [PubMed] [Google Scholar]
- Knoll J. H., Nicholls R. D., Magenis R. E., Glatt K., Graham J. M., Jr, Kaplan L., Lalande M. Angelman syndrome: three molecular classes identified with chromosome 15q11q13-specific DNA markers. Am J Hum Genet. 1990 Jul;47(1):149–154. [PMC free article] [PubMed] [Google Scholar]
- Knoll J. H., Nicholls R. D., Magenis R. E., Graham J. M., Jr, Lalande M., Latt S. A. Angelman and Prader-Willi syndromes share a common chromosome 15 deletion but differ in parental origin of the deletion. Am J Med Genet. 1989 Feb;32(2):285–290. doi: 10.1002/ajmg.1320320235. [DOI] [PubMed] [Google Scholar]
- Knoll J. H., Sinnett D., Wagstaff J., Glatt K., Wilcox A. S., Whiting P. M., Wingrove P., Sikela J. M., Lalande M. FISH ordering of reference markers and of the gene for the alpha 5 subunit of the gamma-aminobutyric acid receptor (GABRA5) within the Angelman and Prader-Willi syndrome chromosomal regions. Hum Mol Genet. 1993 Feb;2(2):183–189. doi: 10.1093/hmg/2.2.183. [DOI] [PubMed] [Google Scholar]
- Kuwano A., Mutirangura A., Dittrich B., Buiting K., Horsthemke B., Saitoh S., Niikawa N., Ledbetter S. A., Greenberg F., Chinault A. C. Molecular dissection of the Prader-Willi/Angelman syndrome region (15q11-13) by YAC cloning and FISH analysis. Hum Mol Genet. 1992 Sep;1(6):417–425. doi: 10.1093/hmg/1.6.417. [DOI] [PubMed] [Google Scholar]
- Ledbetter D. H., Riccardi V. M., Airhart S. D., Strobel R. J., Keenan B. S., Crawford J. D. Deletions of chromosome 15 as a cause of the Prader-Willi syndrome. N Engl J Med. 1981 Feb 5;304(6):325–329. doi: 10.1056/NEJM198102053040604. [DOI] [PubMed] [Google Scholar]
- Leff S. E., Brannan C. I., Reed M. L., Ozçelik T., Francke U., Copeland N. G., Jenkins N. A. Maternal imprinting of the mouse Snrpn gene and conserved linkage homology with the human Prader-Willi syndrome region. Nat Genet. 1992 Dec;2(4):259–264. doi: 10.1038/ng1292-259. [DOI] [PubMed] [Google Scholar]
- Lindeman R., Kouts S., Woodage T., Smith A., Trent R. J. Dinucleotide repeat polymorphism of D15S10 in the Prader-Willi chromosome region (PWCR). Nucleic Acids Res. 1991 Oct 11;19(19):5449–5449. doi: 10.1093/nar/19.19.5449-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magenis R. E., Toth-Fejel S., Allen L. J., Black M., Brown M. G., Budden S., Cohen R., Friedman J. M., Kalousek D., Zonana J. Comparison of the 15q deletions in Prader-Willi and Angelman syndromes: specific regions, extent of deletions, parental origin, and clinical consequences. Am J Med Genet. 1990 Mar;35(3):333–349. doi: 10.1002/ajmg.1320350307. [DOI] [PubMed] [Google Scholar]
- Malcolm S., Clayton-Smith J., Nichols M., Robb S., Webb T., Armour J. A., Jeffreys A. J., Pembrey M. E. Uniparental paternal disomy in Angelman's syndrome. Lancet. 1991 Mar 23;337(8743):694–697. doi: 10.1016/0140-6736(91)90278-w. [DOI] [PubMed] [Google Scholar]
- Mascari M. J., Gottlieb W., Rogan P. K., Butler M. G., Waller D. A., Armour J. A., Jeffreys A. J., Ladda R. L., Nicholls R. D. The frequency of uniparental disomy in Prader-Willi syndrome. Implications for molecular diagnosis. N Engl J Med. 1992 Jun 11;326(24):1599–1607. doi: 10.1056/NEJM199206113262404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijers-Heijboer E. J., Sandkuijl L. A., Brunner H. G., Smeets H. J., Hoogeboom A. J., Deelen W. H., van Hemel J. O., Nelen M. R., Smeets D. F., Niermeijer M. F. Linkage analysis with chromosome 15q11-13 markers shows genomic imprinting in familial Angelman syndrome. J Med Genet. 1992 Dec;29(12):853–857. doi: 10.1136/jmg.29.12.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutirangura A., Greenberg F., Butler M. G., Malcolm S., Nicholls R. D., Chakravarti A., Ledbetter D. H. Multiplex PCR of three dinucleotide repeats in the Prader-Willi/Angelman critical region (15q11-q13): molecular diagnosis and mechanism of uniparental disomy. Hum Mol Genet. 1993 Feb;2(2):143–151. doi: 10.1093/hmg/2.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutirangura A., Kuwano A., Ledbetter S. A., Chinault A. C., Ledbetter D. H. Dinucleotide repeat polymorphism at the D15S11 locus in the Angelman/Prader-Willi region (AS/PWS) of chromosome 15. Hum Mol Genet. 1992 May;1(2):139–139. doi: 10.1093/hmg/1.2.139-a. [DOI] [PubMed] [Google Scholar]
- Mutirangura A., Ledbetter S. A., Kuwano A., Chinault A. C., Ledbetter D. H. Dinucleotide repeat polymorphism at the GABAA receptor beta 3 (GABRB3) locus in the Angelman/Prader-Willi region (AS/PWS) of chromosome 15. Hum Mol Genet. 1992 Apr;1(1):67–67. doi: 10.1093/hmg/1.1.67. [DOI] [PubMed] [Google Scholar]
- Nicholls R. D., Knoll J. H., Butler M. G., Karam S., Lalande M. Genetic imprinting suggested by maternal heterodisomy in nondeletion Prader-Willi syndrome. Nature. 1989 Nov 16;342(6247):281–285. doi: 10.1038/342281a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls R. D., Knoll J. H., Glatt K., Hersh J. H., Brewster T. D., Graham J. M., Jr, Wurster-Hill D., Wharton R., Latt S. A. Restriction fragment length polymorphisms within proximal 15q and their use in molecular cytogenetics and the Prader-Willi syndrome. Am J Med Genet. 1989 May;33(1):66–77. doi: 10.1002/ajmg.1320330109. [DOI] [PubMed] [Google Scholar]
- Ozçelik T., Leff S., Robinson W., Donlon T., Lalande M., Sanjines E., Schinzel A., Francke U. Small nuclear ribonucleoprotein polypeptide N (SNRPN), an expressed gene in the Prader-Willi syndrome critical region. Nat Genet. 1992 Dec;2(4):265–269. doi: 10.1038/ng1292-265. [DOI] [PubMed] [Google Scholar]
- Pembrey M., Fennell S. J., van den Berghe J., Fitchett M., Summers D., Butler L., Clarke C., Griffiths M., Thompson E., Super M. The association of Angelman's syndrome with deletions within 15q11-13. J Med Genet. 1989 Feb;26(2):73–77. doi: 10.1136/jmg.26.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Webb T., Pembrey M. E., Nichols M., Malcolm S. Maternal origin of deletion 15q11-13 in 25/25 cases of Angelman syndrome. Hum Genet. 1992 Feb;88(4):376–378. doi: 10.1007/BF00215668. [DOI] [PubMed] [Google Scholar]
- Wagstaff J., Knoll J. H., Fleming J., Kirkness E. F., Martin-Gallardo A., Greenberg F., Graham J. M., Jr, Menninger J., Ward D., Venter J. C. Localization of the gene encoding the GABAA receptor beta 3 subunit to the Angelman/Prader-Willi region of human chromosome 15. Am J Hum Genet. 1991 Aug;49(2):330–337. [PMC free article] [PubMed] [Google Scholar]
- Wagstaff J., Knoll J. H., Glatt K. A., Shugart Y. Y., Sommer A., Lalande M. Maternal but not paternal transmission of 15q11-13-linked nondeletion Angelman syndrome leads to phenotypic expression. Nat Genet. 1992 Jul;1(4):291–294. doi: 10.1038/ng0792-291. [DOI] [PubMed] [Google Scholar]
- Webb T., Clayton-Smith J., Cheng X. J., Knoll J. H., Lalande M., Pembrey M. E., Malcolm S. Angelman syndrome with a chromosomal inversion 15 inv(p11q13) accompanied by a deletion in 15q11q13. J Med Genet. 1992 Dec;29(12):921–924. doi: 10.1136/jmg.29.12.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C. A., Gray B. A., Hendrickson J. E., Stone J. W., Cantú E. S. Incidence of 15q deletions in the Angelman syndrome: a survey of twelve affected persons. Am J Med Genet. 1989 Mar;32(3):339–345. doi: 10.1002/ajmg.1320320313. [DOI] [PubMed] [Google Scholar]
- Williams C. A., Zori R. T., Stone J. W., Gray B. A., Cantu E. S., Ostrer H. Maternal origin of 15q11-13 deletions in Angelman syndrome suggests a role for genomic imprinting. Am J Med Genet. 1990 Mar;35(3):350–353. doi: 10.1002/ajmg.1320350308. [DOI] [PubMed] [Google Scholar]