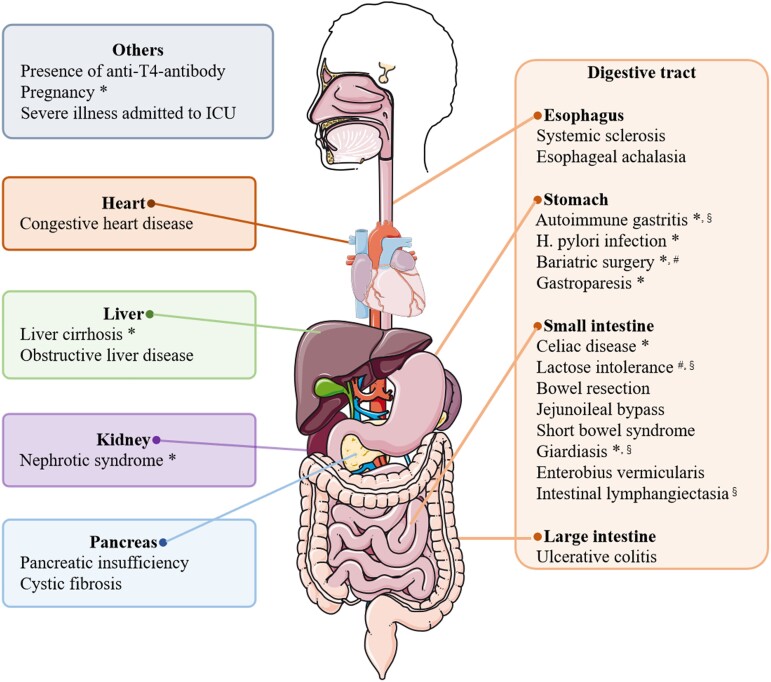
Figure 3.
Summary of concomitant diseases and conditions which interfere with the bioavailability of LT4. *Impaired bioavailability induced by these conditions has been reported to be relieved by novel formulations (liquid solution and/or soft gel capsule). #Malabsorption induced by these conditions has been reported to be relieved by crushed tablet powder. §Malabsorption induced by these conditions has been reported to be relieved by intravenous or intramuscular injection. The figure was partly generated using illustrative elements from Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.