Abstract
The radiotherapy (RT) process from planning to treatment delivery is a multistep, complex operation involving numerous levels of human-machine interaction and requiring high precision. These steps are labor-intensive and time-consuming and require meticulous coordination between professionals with diverse expertise. We reviewed and summarized the current status and prospects of artificial intelligence and machine learning relevant to the various steps in RT treatment planning and delivery workflow specifically in low- and middle-income countries (LMICs). We also searched the PubMed database using the search terms (Artificial Intelligence OR Machine Learning OR Deep Learning OR Automation OR knowledge-based planning AND Radiotherapy) AND (list of Low- and Middle-Income Countries as defined by the World Bank at the time of writing this review). The search yielded a total of 90 results, of which results with first authors from the LMICs were chosen. The reference lists of retrieved articles were also reviewed to search for more studies. No language restrictions were imposed. A total of 20 research items with unique study objectives conducted with the aim of enhancing RT processes were examined in detail. Artificial intelligence and machine learning can improve the overall efficiency of RT processes by reducing human intervention, aiding decision making, and efficiently executing lengthy, repetitive tasks. This improvement could permit the radiation oncologist to redistribute resources and focus on responsibilities such as patient counseling, education, and research, especially in resource-constrained LMICs.
INTRODUCTION
The anticipated increase in cancer burden over the next few years could potentially overwhelm the oncology care system, especially in resource-constrained low- and middle-income countries (LMICs).1,2 Although radiotherapy (RT) is an indispensable component of cancer care, access to it worldwide is very inequitable, with the current density of RT machines per million population ranging from 0 to 11.6, depending on the economic situation of a country.3 The RT process starts with a series of visits to radiation oncology (RO) clinic, culminating in the final diagnosis, staging, and prognostication after which a radiation treatment protocol is assigned. Once a protocol is assigned, the subsequent RT treatment process can be categorized into imaging, target and organs-at-risk (OARs) segmentation, treatment plan generation, onboard imaging, treatment delivery, and quality assurance (QA) checks. These steps are labor-intensive and time-consuming, requiring multiple levels of human-machine interaction and a high degree of precision.4 The patient continues to visit the clinic on conclusion of therapy for toxicity management and follow-up. This workflow is summarized in Figure 1.
FIG 1.
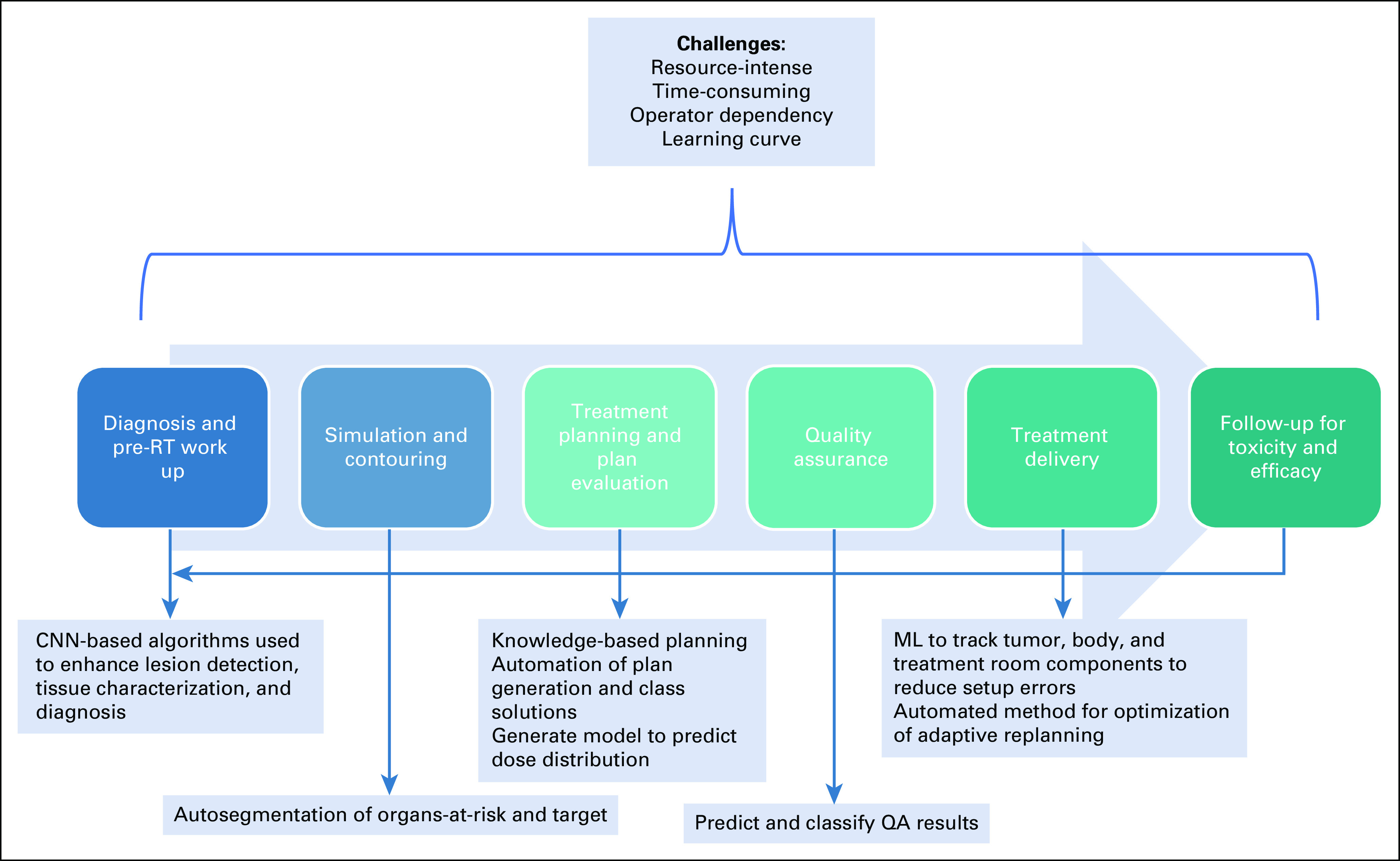
Utility of AI/ML in RT processes. AI, artificial intelligence; CNN, convolutional neural networks; ML, machine learning; QA, quality assurance; RT, radiotherapy.
CONTEXT
Key Objective
We review the role of artificial intelligence (AI) and machine learning (ML) in improving the efficiency of various radiotherapy processes and the challenges in their clinical integration.
Knowledge Generated
AI and ML can improve the accuracy, robustness, and speed of radiotherapy processes by reducing or eliminating human interference, aiding in decision making, and efficiently executing time-consuming, repetitive tasks. As its clinical utility remains yet to be proven, multi-institutional collaborative effort between various stakeholders is urgently needed, before the revolutionary impact of AI and ML bears fruition.
Relevance
The anticipated increase in cancer burden over the next few years coupled with cancer care becoming more personalized and tailored could potentially pressurize the oncology care system in the years to come, especially in low- and middle-income countries.
The RT workflow requires meticulous coordination between trained medical professionals with diverse expertise, i.e., radiation oncologists, medical physicists, dosimetrists, and radiation therapists.5 Understaffing and workforce burnout is, unfortunately, a common problem plaguing RO in LMICs heightened by the ongoing COVID-19 pandemic.6 Training the highly specialized RO workforce requires a high cost and time commitment. The role of artificial intelligence (AI) and machine learning (ML) in optimizing RT processes to achieve the best human, technological, and financial resource utilization is worth exploring.
ARTIFICIAL INTELLIGENCE AND MACHINE LEARNING
Russell and Norvig7 have defined AI as “the designing and building of intelligent agents that receive precepts from the environment and take actions that affect that environment.” A more perceptive definition given by Goel is “the science of building artificial minds by understanding how natural minds work and understanding how natural minds work by building artificial minds.” ML is a branch of AI that allows computer systems to progressively learn, train, and improve on the knowledge gained from a variety of input data without being overtly programed.8 AI and ML can improve the accuracy, robustness, and speed of RT processes by reducing or eliminating human interference, aiding in decision making, and efficiently executing lengthy, repetitive tasks.9 Using ML can free up time for more rewarding tasks such as education, research, patient counseling, and quality checks. The following review focuses on the potential use of ML and AI to transform the existing RT workflow and create a sustainable model that can be adopted in LMICs to supplement human efforts in labor-intensive tasks: segmentation, planning, and QA.
ROLE OF ML IN IMAGE SEGMENTATION
Manual segmentation (or contouring) of the target and OARs is a time-consuming and highly subjective task that lies at the core of RT planning. Historical solutions offered by technology to this conundrum include edge- and region-based methods and atlas-based methods of autosegmentation.10 Deep learning (DL), a subset of ML, is essentially a neural network with three or more layers. These can comprise simple feed-forward models such as artificial neural network or complex models such as convolutional neural networks (CNN) and recurrent neural networks. CNN has been increasingly used to learn complex nonlinear relationships within the imaging data to speed up and improve OARs delineation in mediastinum, pelvis, thorax, brain, and head/neck.11 Image segmentation on the basis of DL uses either patches or regions of an image or the entire image as input to estimate the likelihood that a given image sample belongs to the object being segmented. The likelihood map can be further enhanced by methods combining DL and deformable models.12 Multiple papers published using ML for autosegmentation of OARs have demonstrated no clinically meaningful difference between segmentation by model and clinicians or radiographers with very high values of dice similarity coefficient, while recording a significant reduction in time needed for the segmentation.13-17 For example, the average segmentation time for abdominal OARs liver, stomach, duodenum, and kidneys was 7.1 minutes with automation versus 22.6 minutes when done manually.13 Lesion segmentation is more complex than OARs segmentation because of the heterogeneity in shape, size, and location of the target and, therefore, is still in nascent stages.12 Computer-aided diagnosis methods, including conventional radiomics and CNN-based algorithms that enhance lesion detection in diagnostic radiology, can potentially be used in lesion segmentation during RT planning.18
ROLE OF ML IN RADIOTHERAPY TREATMENT PLAN GENERATION AND ADAPTIVE PLANNING
The advent of intensity-modulated RT and volumetric modulated arc therapy that offer exceptionally conformal RT delivery has increased manifold the intricacy and complexity of RT planning. High-precision treatment procedures such as stereotactic body ablative RT often consume hours or even days of human effort for planning.19 Knowledge-based planning (KBP), which uses data from previous good cases to inform current patient planning parameters, has emerged as a powerful tool to accelerate the process of RT planning.19 Efforts are ongoing to establish indigenous KBP models for cancers common in LMICs, such as cervical cancers, and validate them in various geoethnic populations to test efficacy in patients with different anatomies on the basis of geographical locations.20,21 Supervised DL algorithms have been used for beam direction optimization, where the possible subsequent beam distribution is predicted on the basis of patient anatomy.22 Use of DL in the prediction of spatial dose distribution has been extensively explored, with different architectures of CNN being used to predict the geometric and planning parameters of historical patients.22 A significant gain in time has been reported with ML over non-ML methods such as column generation to select beam orientations, calculate the dose influence matrices, and finally solve the fluence map optimization with comparable dosimetry.23 ML algorithms have also been used to enhance KBP further to generate treatment plans.24 Recent studies have even attempted to emulate the decision-making strategy of human planners when solving a specific dosimetric trade-off problem, thereby potentially reducing the element of subjectivity.25
Another unique approach is the use of Pareto surface–based techniques for multicriteria optimization, where a database of plans is created for a single patient and the plan that achieves the best balance between different treatment planning goals is chosen by the planner and the physician.26,27 The Erasmus i-cycle (created in an academic university) is a vendor-neutral algorithm using multicriteria optimization that is in clinical use for external beam therapy and is being developed for CyberKnife, proton therapy, and brachytherapy (BiCyle).28-32
ROLE OF ML IN RADIOTHERAPY ONBOARD IMAGING AND TREATMENT DELIVERY
ML techniques, including DL approaches, have dealt with intra- and interfraction patient and organ motion during RT treatment delivery to aid tumor gating and motion tracking.33 Frameworks have been built using neural networks trained on collected patient breathing data to predict the breathing pattern while delivering RT.34 ML has been used to aid motion tracking by assisting in the detection of the tumor (marker-less tracking) or surrogate markers.35 ML has been used to help avert setup errors and patient safety hazards by tracking the treatment room components and the patient's body in real time using 3D cameras to fine tune a CNN for object recognition.36 A group of scientists have developed a computer vision–based pneumatic soft robot actuator to better estimate a patient's head pitch motion and to manipulate the patient head position on the basis of sensed head pitch motion, thereby potentially eliminating the need for immobilization with a thermoplastic mask.37
The role of ML in online adaptive RT planning has been extensively explored, mainly in deformable registration and dose warping, facilitating high registration accuracy and efficient execution even if graphical processing units are unavailable.38 A proof-of-concept study investigates online multileaf collimator tracking to generate appropriate safety margins for online adaptation of the treatment plan on the basis of the patient's motion and the ability of the machine to follow these excursions.39 Algorithms can assist physicians in supervising variations during treatment course by evaluating daily setup variations and anatomic changes, for early identification of adaptive replanning requirement.40
ROLE OF ML IN RADIOTHERAPY QUALITY ASSURANCE
Implementing regular and meticulous QA in RT is expected to lead to more accurate treatment delivery and better clinical outcomes. ML has excellent potential to enhance the efficacy and efficiency of RT QA processes as they are often repetitive and time-consuming. ML techniques have been used to predict gamma passing rates and the probability of the plan failing patient-specific intensity-modulated RT QA by analyzing plan complexity; multiple components of the delivery system such as multileaf collimator, imaging system, and mechanical and dosimetric parameters, and plan delivery log files over time.41-43 Another approach has identified RT treatment delivery errors using radiomics-based feature extraction from patient-specific gamma images.44 A study has applied artificial neural network–based time series prediction modeling to predict the performance of beam symmetry of linear accelerators over time.45 Although these in silico approaches of various ML tools have augmented RT QA procedures, it is pertinent to establish its real utility in the clinical context before implementation.
OTHER APPLICATIONS OF AI IN RADIATION ONCOLOGY
Data Annotation, Radiomics, and Response Prediction
Radiomics is a method that extracts a large number of features from medical images using data characterization algorithms.46 Many institutions and health networks, including from India, are working to create repositories of annotated medical data and medical images including outcomes of treatment for furthering radiomic research in large image data sets.47,48 Distributed learning approaches with AI support have been used to conduct population-based studies on routine data and build decision support models.49,50 Image banking combined with predictive/prescriptive AI is a cost-effective and efficient alternative to identify signatures for response, toxicity, and outcome prediction after cancer treatment.51-53
Natural Language Processing
Natural language processing (NLP) is a branch of AI that enables computers to interpret human language. NLP has already found application in the medical world to facilitate data extraction from free text in electronic medical records. The specific utility of NLP being explored in RO is standardization of contours and plans nomenclature to enable efficient data extraction.54
ORIGINAL RESEARCH FROM LMICS USING AUTOMATION AND ML TO OPTIMIZE RT PLANNING, TREATMENT DELIVERY, AND QA PROCESSES
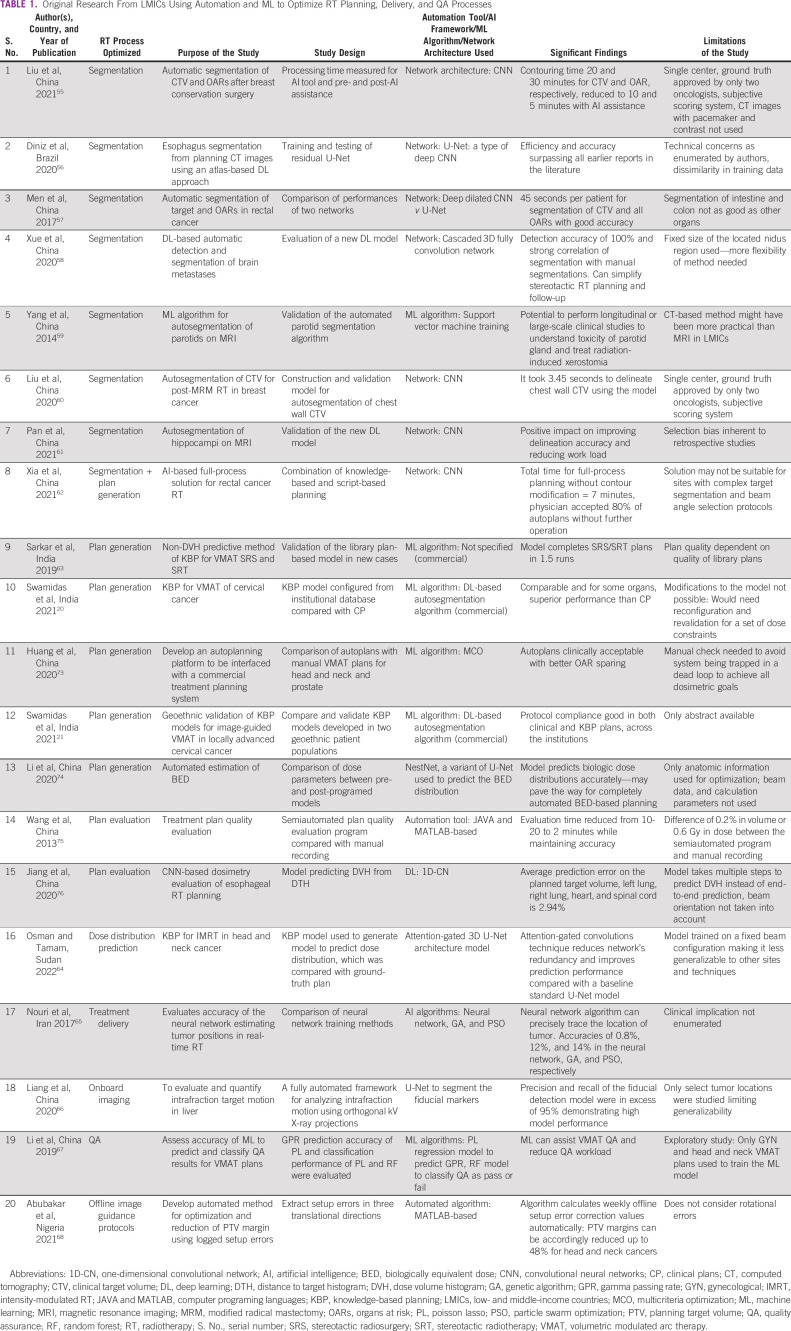
We searched the PubMed database using the search term (Artificial Intelligence[tw] OR Machine Learning[tw] OR Deep Learning[tw] OR Automation[tw] automated[tw] OR knowledge-based planning[tw] AND Radiotherapy[tw]) AND (list of Low- and Middle-Income Countries as defined by the World Bank at the time of writing this review). The search yielded a total of 90 results, of which results with first authors from the LMICs were chosen. The reference lists of retrieved articles were also reviewed to search for more studies. No language restrictions were imposed. A total of 20 research items with unique study objectives conducted with the aim of enhancing RT processes were studied in detail and are presented in Table 1.
TABLE 1.
Original Research From LMICs Using Automation and ML to Optimize RT Planning, Delivery, and QA Processes
The majority of studies have focused on the utilization of CNN and other networks for autosegmentation.55-62 The striking reduction in time burden seen with incorporating these algorithms while maintaining the accuracy of contours can prove to be pivotal in resource allocation in LMICs. The studies on autoplanning have mainly used KBP.20,21,62,63 Developing indigenous models with local data rather than adopting western models to fit into the local workflow seems to be the standard approach, which is undoubtedly remarkable. Studies from LMICs using AI/ML to assist in online adaptive planning, treatment delivery, and QA are few and far between, and more work in this area must be encouraged.64,66‐68
CHALLENGES OF INTEGRATING AUTOMATION AND ML INTO CLINICAL PROCESSES
Although AI has already become pervasive in our day-to-day activities and has the potential to influence how medicine is practiced, many challenges remain before its complete integration into RT processes, as listed below.69
Clinical utility yet to be determined: Most ML-based solutions are still in the stage of technological incubation, with the onus on the RO team to establish their clinical value.
Risk analysis of automation and AI: QA studies for automated treatment planning tools have been conducted, which stress the need for comprehensive manual review of the plans by physicians and physicists before implementation.70
Black-box nature of AI algorithms: In the case of failure of AI-based solutions, there is no straightforward framework to fix the outcome or predict errors. This lack of transparency and difficulty in understanding the outputs and predicting failures may make physicians hesitant and distrustful to rely on AI in patient-related decisions, further delaying the adoption of AI into clinical practice. It is essential to train the RO staff to correctly use the ML model and accurately interpret the intended utility, scope, and limitations.
Interpretation: It is necessary to remember that even if some algorithms can perform at near-human ability, the way they perceive and interpret the inputs differs from the human mind.
Training data: A machine learning algorithm's accuracy and generalizability are influenced heavily by the quality and quantity of the training data more than the mathematical parameters. Since individual institutional data sets are bound to be minor, data sharing across multiple institutions can make the ML/DL algorithms more robust. Distributed learning is an emerging approach to securely transferring data sets between institutions.71
Patient privacy and anonymity: The potential of distributed learning to provide evidence-based personalized care in LMICs is immense. However, care should be taken to uphold the rules of ethics, standardization, and stringent privacy regulations.72
In conclusion, integrating AI and ML in RT processes may allow radiation oncologists to spend more time on patient consultation, teaching, and research in resource-constrained setups with a heavy workload. Given the transformative impact that AI-based technology can bring to clinical processes and workflow, it is essential to integrate these concepts early in medical education and RO residency to facilitate a better understanding of the methods and encourage innovation. We have in front of us a means to revolutionize the practice of RT as we know it. It is our responsibility toward the future generation to understand, plan, prioritize, conduct meaningful research, integrate and constantly improve AI and ML in RT without bias or prejudice, and deliver it to settings where the impact would be maximum.
Supriya Chopra
This author is an Associate Editor for JCO Global Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Varian Medical Systems, KM Pharmaceuticals (KORTUC) (Inst)
Consulting or Advisory Role: KM Pharmaceuticals (KORTUC) (Inst)
Speakers' Bureau: Varian Medical Systems (Inst)
Research Funding: Varian Medical Systems, Elekta (Inst)
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Supriya Chopra
This author is an Associate Editor for JCO Global Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Varian Medical Systems, KM Pharmaceuticals (KORTUC) (Inst)
Consulting or Advisory Role: KM Pharmaceuticals (KORTUC) (Inst)
Speakers' Bureau: Varian Medical Systems (Inst)
Research Funding: Varian Medical Systems, Elekta (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. : Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Zubizarreta EH, Fidarova E, Healy B, et al. : Need for radiotherapy in low- and middle-income countries—The silent crisis continues. Clin Oncol 27:107-114, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Wahab M, Gondhowiardjo SS, Rosa AA, et al. : Global radiotherapy: Current status and future directions-white paper. JCO Glob Oncol 7:827-842, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bortfeld T, Jeraj R: The physical basis and future of radiation therapy. Br J Radiol 84:485-498, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valentini V, Boldrini L, Mariani S, Massaccesi M: Role of radiation oncology in modern multidisciplinary cancer treatment. Mol Oncol 14:1431-1441, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wadasadawala T, Kumar A, Laskar SG, et al. : Multinational study to assess stress levels among the health care workers of radiation oncology community at the outset of the COVID-19 pandemic. JCO Glob Oncol 7:464-473, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell SJ, Norvig P: Artificial Intelligence: A Modern Approach (ed 4). Hoboken, Pearson, 2021 [Google Scholar]
- 8.Nichols JA, Herbert Chan HW, Baker MAB: Machine learning: Applications of artificial intelligence to imaging and diagnosis. Biophys Rev 11:111-118, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davenport T, Kalakota R: The potential for artificial intelligence in healthcare. Future Healthc J 6:94-98, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalet IJ, Paluszynski W: Knowledge-based computer systems for radiotherapy planning. Am J Clin Oncol 13:344-351, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Boldrini L, Bibault JE, Masciocchi C, et al. : Deep learning: A review for the radiation oncologist. Front Oncol 9:977, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahiner B, Pezeshk A, Hadjiiski LM, et al. : Deep learning in medical imaging and radiation therapy. Med Phys 46:e1-e36, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Racine D, Becce F, Viry A, et al. : Task-based characterization of a deep learning image reconstruction and comparison with filtered back-projection and a partial model-based iterative reconstruction in abdominal CT: A phantom study. Phys Med 76:28-37, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Nikolov S, Blackwell S, Zverovitch A, et al. : Clinically applicable segmentation of head and neck anatomy for radiotherapy: Deep learning algorithm development and validation study. J Med Internet Res 23:e26151, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter E, Fuentes P, Siddiqui Z, et al. : Hippocampus segmentation on noncontrast CT using deep learning. Med Phys 47:2950-2961, 2020 [DOI] [PubMed] [Google Scholar]
- 16.van Velzen SGM, Bruns S, Wolterink JM, et al. : AI-based quantification of planned radiotherapy dose to cardiac structures and coronary arteries in breast cancer patients. Int J Radiat Oncol Biol Phys 112:611-620, 2022 [DOI] [PubMed] [Google Scholar]
- 17.Rigaud B, Anderson BM, Yu ZH, et al. : Automatic segmentation using deep learning to enable online dose optimization during adaptive radiation therapy of cervical cancer. Int J Radiat Oncol Biol Phys 109:1096-1110, 2021 [DOI] [PubMed] [Google Scholar]
- 18.Giger ML, Chan HP, Boone J: Anniversary paper: History and status of CAD and quantitative image analysis: The role of medical physics and AAPM. Med Phys 35:5799-5820, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore KL: Automated radiotherapy treatment planning. Semin Radiat Oncol 29:209-218, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Swamidas J, Pradhan S, Chopra S, et al. : Development and clinical validation of knowledge-based planning for volumetric modulated arc therapy of cervical cancer including pelvic and para aortic fields. Phys Imaging Radiat Oncol 18:61-67, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swamidas J, Assenholt M, Serban M, et al. : PO-1855 Protocol compliance of two knowledge based models in two geo-ethnic populations for cervical cancer. Radiother Oncol 161:S1581, 2021 [Google Scholar]
- 22.Wang M, Zhang Q, Lam S, et al. : A review on application of deep learning algorithms in external beam radiotherapy automated treatment planning. Front Oncol 10:580919, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadeghnejad Barkousaraie A, Ogunmolu O, Jiang S, et al. : A fast deep learning approach for beam orientation optimization for prostate cancer treated with intensity-modulated radiation therapy. Med Phys 47:880-897, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Zhang J, Sheng Y, et al. : Automatic IMRT planning via static field fluence prediction (AIP-SFFP): A deep learning algorithm for real-time prostate treatment planning. Phys Med Biol 65:175014, 2020 [DOI] [PubMed] [Google Scholar]
- 25.Shen C, Nguyen D, Chen L, et al. : Operating a treatment planning system using a deep-reinforcement learning-based virtual treatment planner for prostate cancer intensity-modulated radiation therapy treatment planning. Med Phys 47:2329-2336, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craft DL, Hong TS, Shih HA, et al. : Improved planning time and plan quality through multicriteria optimization for intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 82:e83-e90, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Zhang Q, Lam S, et al. : A review on application of deep learning algorithms in external beam radiotherapy automated treatment planning. Front Oncol 10:580919, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossi L, Breedveld S, Heijmen BJM, et al. : On the beam direction search space in computerized non-coplanar beam angle optimization for IMRT-prostate SBRT. Phys Med Biol 57:5441-5458, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Rossi L, Sharfo AW, Aluwini S, et al. : First fully automated planning solution for robotic radiosurgery—Comparison with automatically planned volumetric arc therapy for prostate cancer. Acta Oncol 57:1490-1498, 2018 [DOI] [PubMed] [Google Scholar]
- 30.van de Water S, Kooy HM, Heijmen BJM, et al. : Shortening delivery times of intensity modulated proton therapy by reducing proton energy layers during treatment plan optimization. Int J Radiat Oncol Biol Phys 92:460-468, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Breedveld S, Bennan ABA, Aluwini S, et al. : Fast automated multi-criteria planning for HDR brachytherapy explored for prostate cancer. Phys Med Biol 64:205002, 2019 [DOI] [PubMed] [Google Scholar]
- 32.Oud M, Kolkman-Deurloo IK, Mens JW, et al. : Fast and fully-automated multi-criterial treatment planning for adaptive HDR brachytherapy for locally advanced cervical cancer. Radiother Oncol 148:143-150, 2020 [DOI] [PubMed] [Google Scholar]
- 33.Lin T, Li R, Tang X, et al. : Markerless gating for lung cancer radiotherapy based on machine learning techniques. Phys Med Biol 54:1555-1563, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Terunuma T, Tokui A, Sakae T: Novel real-time tumor-contouring method using deep learning to prevent mistracking in X-ray fluoroscopy. Radiol Phys Technol 11:43-53, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mylonas A, Keall PJ, Booth JT, et al. : A deep learning framework for automatic detection of arbitrarily shaped fiducial markers in intrafraction fluoroscopic images. Med Phys 46:2286-2297, 2019 [DOI] [PubMed] [Google Scholar]
- 36.Santhanam A, Min Y, Beron P, et al. : Low SU-D-201-05: On the automatic recognition of patient safety hazards in a radiotherapy setup using a novel 3D camera system and a deep learning framework. Med Phys 43:3334-3335, 2016 [Google Scholar]
- 37.Ogunmolu OP, Gu X, Jiang S, et al. : Vision-based control of a soft robot for maskless head and neck cancer radiotherapy. August 21-24, 2016, Fort Worth, TX, 2016 IEEE Int Conf Autom Sci Eng:180-187, 2016 [Google Scholar]
- 38.Xiao H, Ren G, Cai J: A review on 3D deformable image registration and its application in dose warping. Radiat Med Prot 1:171-178, 2020 [Google Scholar]
- 39.Glitzner M, Fast MF, de Senneville BD, et al. : Real-time auto-adaptive margin generation for MLC-tracked radiotherapy. Phys Med Biol 62:186-201, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guidi G, Maffei N, Meduri B, et al. : A machine learning tool for re-planning and adaptive RT: A multicenter cohort investigation. Phys Med 32:1659-1666, 2016 [DOI] [PubMed] [Google Scholar]
- 41.Valdes G, Chan MF, Lim SB, et al. : IMRT QA using machine learning: A multi-institutional validation. J Appl Clin Med Phys 18:279-284, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam D, Zhang X, Li H, et al. : Predicting gamma passing rates for portal dosimetry-based IMRT QA using machine learning. Med Phys 46:4666-4675, 2019 [DOI] [PubMed] [Google Scholar]
- 43.Osman AF, Maalej NM, Jayesh K: Prediction of the individual multileaf collimator positional deviations during dynamic IMRT delivery priori with artificial neural network. Med Phys 47:1421-1430, 2020 [DOI] [PubMed] [Google Scholar]
- 44.Ma C, Wang R, Zhou S, et al. : The structural similarity index for IMRT quality assurance: Radiomics-based error classification. Med Phys 48:80-93, 2021 [DOI] [PubMed] [Google Scholar]
- 45.Li Q, Chan MF: Predictive time series modeling using artificial neural networks for linac beam symmetry – An empirical study. Ann N Y Acad Sci 1387:84-94, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agarwal JP, Sinha S, Goda JS, et al. : Tumor radiomic features complement clinico-radiological factors in predicting long-term local control and laryngectomy free survival in locally advanced laryngo-pharyngeal cancers. Br J Radiol 93:20190857, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kundu S, Chakraborty S, Mukhopadhyay J, et al. : Research goal-driven data model and harmonization for de-identifying patient data in radiomics. J Digit Imaging 34:986-1004, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chopra S: Development of Clinically High Efficient Platforms for Individualised Treatment of Cervix Cancer. https://clinicaltrials.gov/ct2/show/NCT05102240 [Google Scholar]
- 49.Field M, Vinod S, Aherne N, et al. : Implementation of the Australian Computer-Assisted Theragnostics (AusCAT) network for radiation oncology data extraction, reporting and distributed learning. J Med Imaging Radiat Oncol 65:627-636, 2021 [DOI] [PubMed] [Google Scholar]
- 50.Choudhury A, Theophanous S, Lønne P-I, et al. : Predicting outcomes in anal cancer patients using multi-centre data and distributed learning—A proof-of-concept study. Radiother Oncol 159:183-189, 2021 [DOI] [PubMed] [Google Scholar]
- 51.Yakar M, Etiz D, Metintas M, et al. : Prediction of radiation pneumonitis with machine learning in stage III lung cancer: A pilot study. Technol Cancer Res Treat 20:15330338211016373, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin C, Yu H, Ke J, et al. : Predicting treatment response from longitudinal images using multi-task deep learning. Nat Commun 12:1851, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel M, Zhan J, Natarajan K, et al. : Machine learning-based radiomic evaluation of treatment response prediction in glioblastoma. Clin Radiol 76:628.e17-628.e27, 2021 [DOI] [PubMed] [Google Scholar]
- 54.Bitterman DS, Miller TA, Mak RH, et al. : Clinical natural language processing for radiation oncology: A review and practical primer. Int J Radiat Oncol Biol Phys 110:641-655, 2021 [DOI] [PubMed] [Google Scholar]
- 55.Liu Z, Liu F, Chen W, et al. : Automatic segmentation of clinical target volume and organs-at-risk for breast conservative radiotherapy using a convolutional neural network. Cancer Manag Res 13:8209-8217, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diniz JOB, Ferreira JL, Diniz PHB, et al. : Esophagus segmentation from planning CT images using an atlas-based deep learning approach. Comput Methods Programs Biomed 197:105685, 2020 [DOI] [PubMed] [Google Scholar]
- 57.Men K, Dai J, Li Y: Automatic segmentation of the clinical target volume and organs at risk in the planning CT for rectal cancer using deep dilated convolutional neural networks. Med Phys 44:6377-6389, 2017 [DOI] [PubMed] [Google Scholar]
- 58.Xue J, Wang B, Ming Y, et al. : Deep learning-based detection and segmentation-assisted management of brain metastases. Neuro Oncol 22:505-514, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang X, Wu N, Cheng G, et al. : Automated segmentation of the parotid gland based on atlas registration and machine learning: A longitudinal MRI study in head-and-neck radiation therapy. Int J Radiat Oncol Biol Phys 90:1225-1233, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Z, Liu F, Chen W, et al. : Automatic segmentation of clinical target volumes for post-modified radical mastectomy radiotherapy using convolutional neural networks. Front Oncol 10:581347, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pan K, Zhao L, Gu S, et al. : Deep learning-based automatic delineation of the hippocampus by MRI: Geometric and dosimetric evaluation. Radiat Oncol 16:12, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xia X, Wang J, Li Y, et al. : An artificial intelligence-based full-process solution for radiotherapy: A proof of concept study on rectal cancer. Front Oncol 10:616721, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sarkar B, Munshi A, Ganesh T, et al. : Standardization of volumetric modulated arc therapy-based frameless stereotactic technique using a multidimensional ensemble-aided knowledge-based planning. Med Phys 46:1953-1962, 2019 [DOI] [PubMed] [Google Scholar]
- 64.Osman AFI, Tamam NM: Attention-aware 3D U-Net convolutional neural network for knowledge-based planning 3D dose distribution prediction of head- and-neck cancer. J Appl Clin Med Phys 23:e13630, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nouri S, Hosseini Pooya SM, Soltani Nabipour J: Comparative analysis of neural network training methods in real-time radiotherapy. J Biomed Phys Eng 7:51-58, 2017 [PMC free article] [PubMed] [Google Scholar]
- 66.Liang Z, Zhou Q, Yang J, et al. : Artificial intelligence-based framework in evaluating intrafraction motion for liver cancer robotic stereotactic body radiation therapy with fiducial tracking. Med Phys 47:5482-5489, 2020 [DOI] [PubMed] [Google Scholar]
- 67.Li J, Wang L, Zhang X, et al. : Machine learning for patient-specific quality assurance of VMAT: Prediction and classification accuracy. Int J Radiat Oncol Biol Phys 105:893-902, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abubakar A, Zamri NAM, Shaukat SI, et al. : Automated algorithm for calculation of setup corrections and planning target volume margins for offline image-guided radiotherapy protocols. J Appl Clin Med Phys 22:137-146, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Netherton TJ, Cardenas CE, Rhee DJ, et al. : The emergence of artificial intelligence within radiation oncology treatment planning. Oncology 99:124-134, 2021 [DOI] [PubMed] [Google Scholar]
- 70.Kisling K, Johnson JL, Simonds H, et al. : A risk assessment of automated treatment planning and recommendations for clinical deployment. Med Phys 46:2567-2574, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kiser KJ, Fuller CD, Reed VK: Artificial intelligence in radiation oncology treatment planning: A brief overview. J Med Artif Intell 2:9, 2019 [Google Scholar]
- 72.Yirmibesoglu Erkal E, Akpınar A, Erkal HŞ: Ethical evaluation of artificial intelligence applications in radiotherapy using the Four Topics Approach. Artif Intell Med 115:102055, 2021 [DOI] [PubMed] [Google Scholar]
- 73.Huang X, Quan H, Zhao B, et al. : A plan template-based automation solution using a commercial treatment planning system. J Appl Clin Med Phys 21:13-25, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y, He K, Ma M, et al. : Using deep learning to model the biological dose prediction on bulky lung cancer patients of partial stereotactic ablation radiotherapy. Med Phys 47:6540-6550, 2020 [DOI] [PubMed] [Google Scholar]
- 75.Wang J, Chen W, Studenski M, et al. : A semi-automated tool for treatment plan-quality evaluation and clinical trial quality assurance. Phys Med Biol 58:N181-N187, 2013 [DOI] [PubMed] [Google Scholar]
- 76.Jiang D, Yan H, Chang N, et al. : Convolutional neural network-based dosimetry evaluation of esophageal radiation treatment planning. Med Phys 47:4735-4742, 2020 [DOI] [PubMed] [Google Scholar]