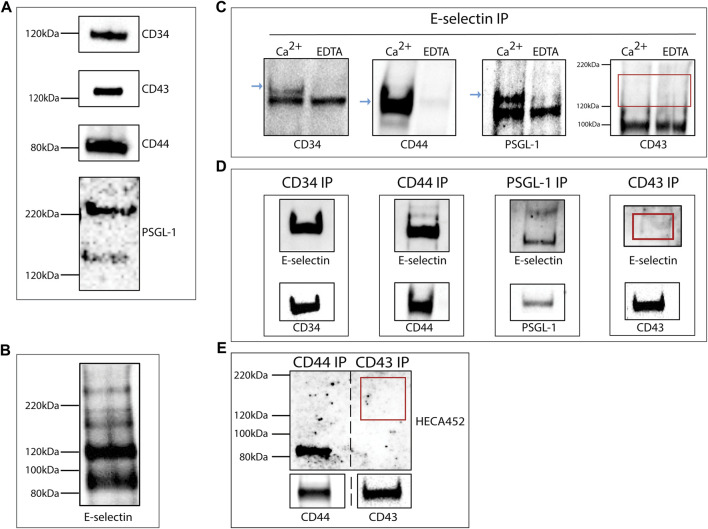
FIGURE 2.
KG1a-derived exosomes express E-selectin ligands. (A) Western blot analysis of lysates of KG1a-derived exosomes revealed the presence of CD34, CD43, CD44, and PSGL-1 at the expected molecular weights. For PSGL-1, two bands were detected as expected (Snapp et al., 1998), a lower band corresponding to the monomeric form and a higher band corresponding to the dimeric form. (B) Western blot analysis of KG1a-derived exosome lysates stained with rE-selectin-IgG revealed several potential E-selectin ligands. (C) rE-selectin-IgG was used to immunoprecipitate (IP) proteins from lysates of KG1a-derived exosomes either in the presence of Ca2+ (2 mM) or EDTA (20 mM). Western blot analysis revealed the presence of CD34, CD44, and PSGL-1 (blue arrows) in samples where Ca2+ was added but not in samples containing EDTA confirming the selective, calcium-dependent binding of E-selectin to its ligands. In western blots for CD43, no band was observed at the expected MW (see red rectangle). (D) CD44, CD34, PSGL-1, and CD43 were immunoprecipitated (IP) from the lysates of KG1a-derived exosomes using antibodies against each potential E-selectin ligand. The IP product was blotted against the rE-selectin-IgG (upper) and each respective ligand (lower, positive control). A clear band corresponding to CD43 was not detected (see red rectangle). Note that at in the upper blots, double the amount of sample was loaded compared to the lower blots in order to establish that although a substantial amount of CD43 protein is present (lower), the lack of E-selectin binding (upper) is likely a result of the lack of proper glycosylation. (E) CD44 and CD43 were Immunoprecipitated (IP) from lysates of KG1a-derived exosomes using antibodies against each ligand. The IP product was blotted for sLex/a using the HECA-452 antibody (upper) and each specific ligand (lower). No band corresponding to CD43 was detected with HECA-452 (see red rectangle) even though CD43 was present (see lower CD43 blot) suggesting that this ligand does not express sLex/a structures. Results shown are representative of n = 3 independent experiments.