FIGURE 1.
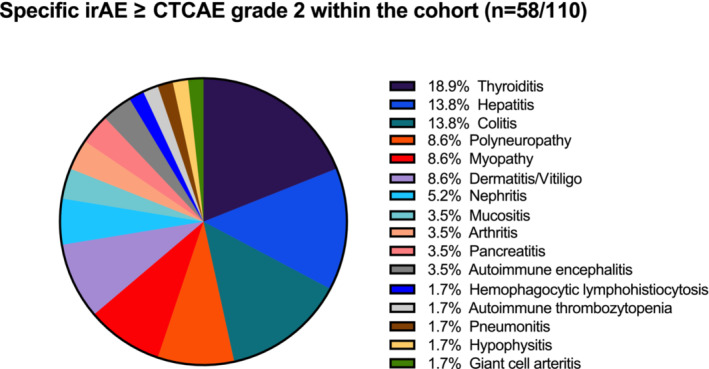
Illustration of specific immune‐related adverse events (irAE) including neurological adverse events (nAE) within the total cohort (n = 58/110, 52.7%). Displayed are specific ICI‐induced autoimmune adverse events of ≥CTCAE grade 2 including specific nAE. CTCAE, common criteria terminology for adverse events. Version 6.0 CTCAE was used.
