FIGURE 3.
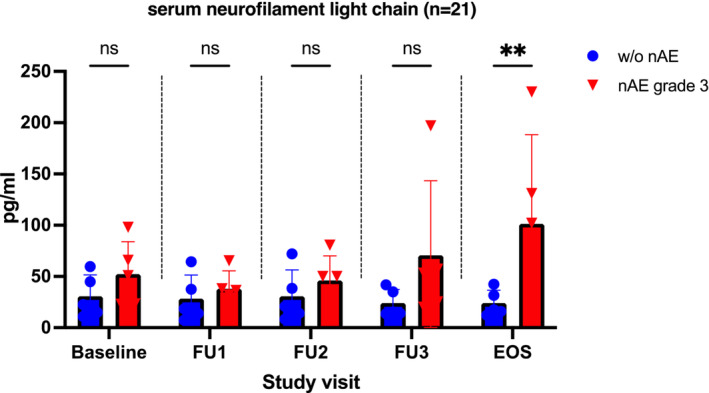
Comparison of neurofilament light chain in serum in patients without nAE and those with nAE grade 3. EOS, end‐of‐study; FU, follow‐up; nAE, neurological adverse events; ns, not significant. ** p < 0.01. At each time point/study visit, n = 5 patients from each group were studied. None of the patients with nAE showed neurological symptoms at baseline.
