FIGURE 4.
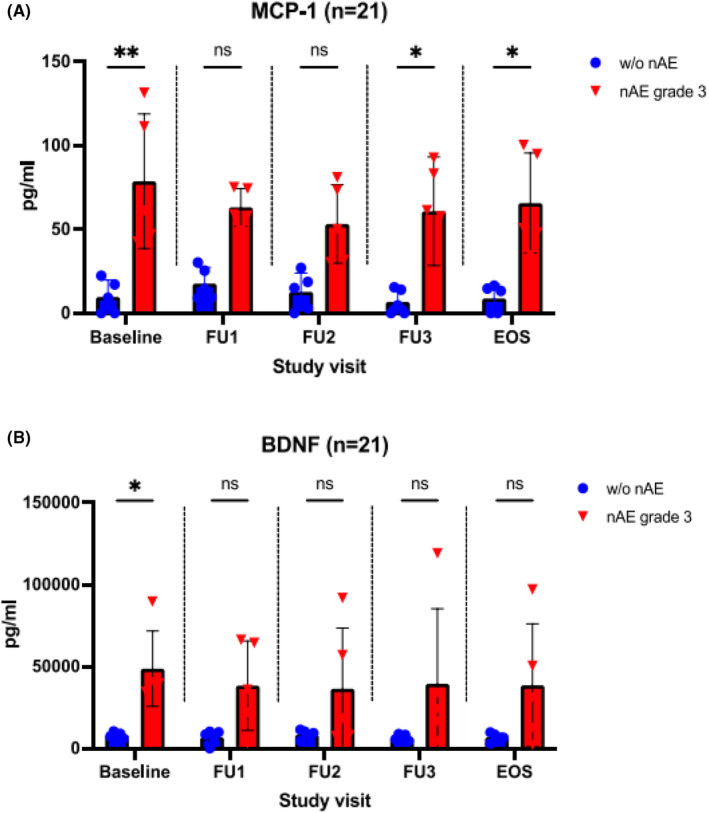
Cytokine measurement in patients with and without ICI‐associated neurotoxicity CTCAE grade 3. (A) Serum monocyte chemoattractant protein 1 (MCP‐1) concentrations, comparison of patients without nAE and those with nAE grade 3. (B) Serum levels of brain derived neurotrophic factor (BDNF), comparison of patients without nAE and those with nAE grade 3. EOS, end‐of‐study; FU, follow‐up; nAE, neurological adverse events; ns, not significant. ** p < 0.01, * p < 0.05. At each time point/study visit, n = 5 patients from each group were studied. None of the patients with nAE showed neurological symptoms at baseline.
