Fig. 2. Rotation of the LPC headgroup.
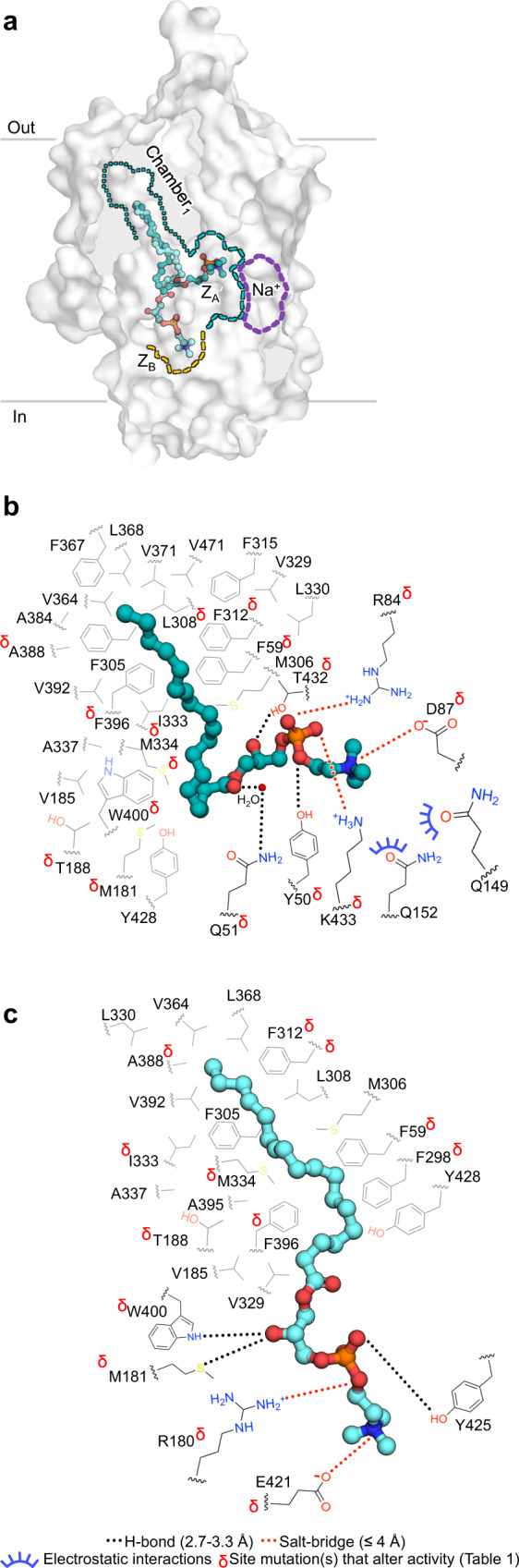
a Relative positions of Lysolipid1A-1B in Chamber1 and ZA-B. Lipid Chamber1 is outlined in cyan dotted lines. ZA-B are in dashed cyan and orange lines, respectively. The proposed Na+ binding site is highlighted by dashed purple lines. Protein rendered as surface. Lysolipids1A-1B are in stick and sphere. b Interactions between lipid tail and LPC of Lysolipid1A with residues in Chamber1 and ZA. c Interactions between lipid tail and LPC of Lysolipid1B with residues in Chamber1 and ZB. b, c Carbons of lipid chamber residues are in gray. Carbons of Z-site residues are in black. Black dotted lines represent H-bonding between 2.6 Å and 3.3 Å. Red dotted lines indicate salt-bridges with distances ≤4 Å. Blue half circles indicate choline coordinating residues within 3.5 Å. Red δ indicate residues with mutations that alter activity (Table 1). Water molecule shown as small red sphere.
