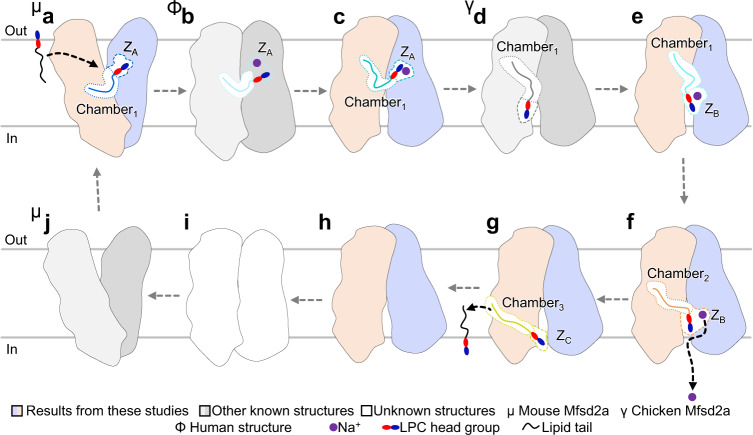
Fig. 4. Proposed model for Mfsd2a lipid transport cycle.
a The ligand-bound, outward-facing mouse Mfsd2a structure docked with ALA-LPC is bound in a lateral position Chamber1 (see Supplementary discussion). The LPC headgroup is trapped by ZA in an outward pointing orientation. b The proposed and unknown occluded ligand-bound conformation. Mfsd2a rocks from an outward to inward-facing conformation. c–e The various conformations during flipping of the LPC headgroup. The lipid tail is held in position by Chamber1 while the LPC headgroup samples multiple configurations between ZA and ZB before it is moved to ZB. This movement flips the LPC headgroup from an outward to inward-pointing orientation. This process results in a substrate with the lipid tail pointing out and the LPC headgroup pointing in, a ligand configuration that is aligned with the inner membrane leaflet. f The translocation of the lysolipid to Chamber2 and ZB. The lipid tail is translocated from Chamber1 to Chamber2. The LPC headgroup is shifted inwards. This process translocates the entire lipid-LPC substrate closer to the cytoplasmic exit. g The release of the substrate to the cytoplasmic side. LPC headgroup is moved to ZC and the lipid tail is translocated to Chamber3. Chamber3 and ZC are located at the cytoplasmic exit. h The substrate-free structure of drMfsd2a in the inward-facing conformation. This structure represents the conformation after lysolipid release from Mfsd2a. i–j The conformations during resetting of the transporter. In the absence of the substrate, Mfsd2a resets to the outward-open conformation (j) by first transitioning through a ligand-free occluded state (i).