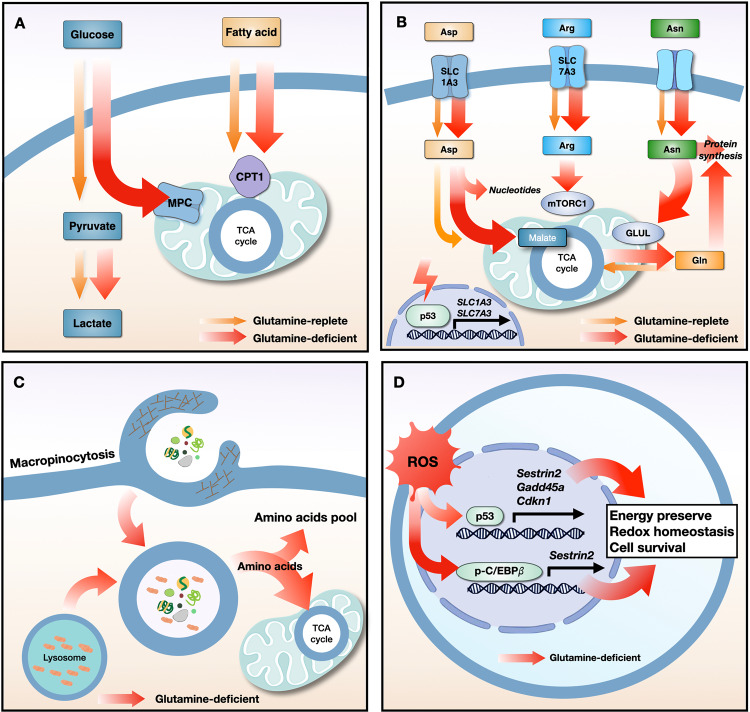
Fig. 2. Resistance mechanisms used by cancer cells in response to glutamine starvation.
a Glutamine starvation induces metabolic flexibility, in which the influx of glucose-derived pyruvate via MPC and fatty acid-derived acyl-CoA via CPT1 into the mitochondria drives TCA cycle activity. b Under conditions of glutamine deprivation, the tumor suppressor protein p53 induces the expression of the SLC1A3 and SLC7A2 transporters. Aspartate uptake through SLC1A3 transporters increases the amount of malate, which is a TCA cycle intermediate, leading to an increase in oxidative phosphorylation and glutamine synthesis. Aspartate is used for nucleotide synthesis. Arginine uptake through SLC7A3 transporters restores mTORC1 expression, which is suppressed by glutamine depletion. The high level of intracellular asparagine increases the expression of GLUL proteins, thereby increasing glutamine and protein synthesis. c Under conditions of nutrient stress, macropinocytosis internalizes extracellular macromolecules to supply amino acids. Membrane ruffling aids in the uptake of extracellular macromolecules, such as serum albumin, via the formation of macropinosomes. After fusion between macropinosomes and lysosomes, albumin is degraded to supply amino acids to the cytosol and the mitochondrial TCA cycle. d Glutamine deprivation increases the expression of p53 and its target genes (Sestrin2, Gadd45a, and Cdkn1) and increases the phosphorylation of C/EBPβ and its target gene (Sestrin2), all of which maintain energy and redox balance and increase cancer cell survival. MPC, mitochondrial pyruvate carrier; CPT1, carnitine palmitoyltransferase I; TCA, tricarboxylic acid cycle; Asp, aspartate; Arg, arginine; Asn, asparagine; Gln, glutamine; ROS, reactive oxygen species; GLUL, glutamate-ammonia ligase; C/EBPβ, CCAAT/enhancer binding protein β.