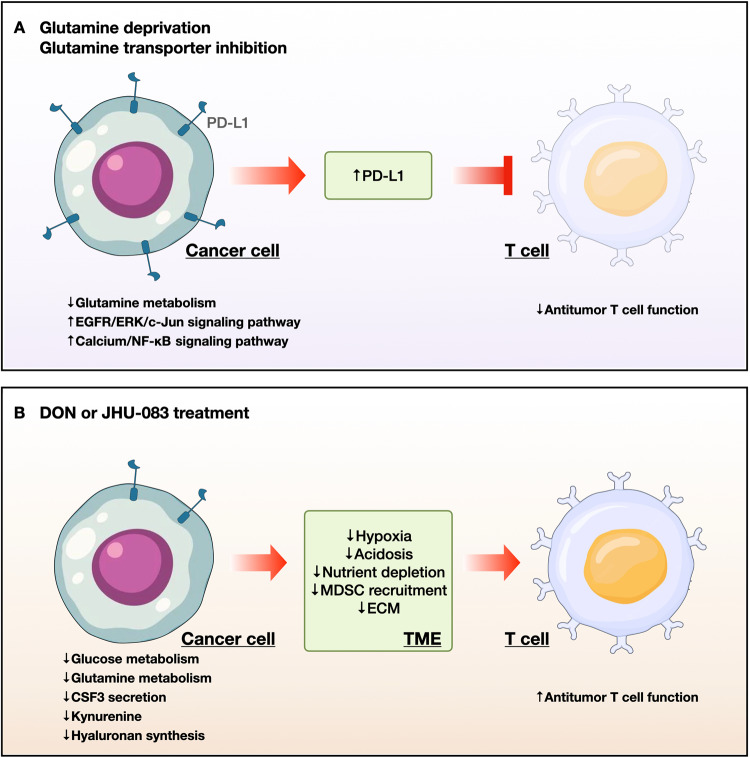
Fig. 3. T-cell-mediated immune responses to glutamine-targeted treatment in cancer cells.
a Glutamine deprivation and transporter inhibition decrease glutamine metabolism, thereby boosting EGFR/ERK/c-Jun signaling and calcium/NF-kB signaling, leading to upregulation of PD-L1. PD-L1 suppresses antitumor immune responses by blocking T-cell activation in the tumor microenvironment. b Treatment with glutamine analogs, including DON and JHU-083, decreases glucose and glutamine metabolism, leading to inhibition of tumor growth via a decrease in hypoxia, acidosis, and nutrient depletion in the tumor microenvironment. Furthermore, DON decreases the recruitment of MDSCs by suppressing the secretion of CSF3 by tumor cells and blocking the production of the immunosuppressive metabolite kynurenine; this inhibits the synthesis of the hyaluronan-rich ECM, resulting in the activation and infiltration of T cells. PD-L1, programmed death-ligand 1; CSF3, colony stimulating factor 3; MDSC, myeloid-derived suppressor cell; ECM, extracellular matrix.