Abstract
We have examined the ability of peptidoglycan (PepG) and lipoteichoic acid (LTA) isolated from Staphylococcus aureus to induce the release of tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-10 in whole human blood and identified the cellular origins of these cytokines. Both PepG and LTA induced transient increases in TNF-α and IL-10 in plasma, with peak values at 6 and 12 h, respectively. IL-6 values increased throughout the experimental period (24 h). The TNF-α, IL-6, and IL-10 release induced by PepG and LTA was dose dependent. Only PepG was a potent inducer of TNF-α secretion. After stimulation of whole blood with PepG or LTA, very pure populations of monocytes (CD14 positive), T cells (CD2 positive), B cells (CD19 positive), and granulocytes (CD15 positive) were isolated by immunomagnetic separation and analyzed by reverse transcription-PCR for mRNA transcripts encoding TNF-α, IL-6, and IL-10. The TNF-α mRNA results were inconclusive. In contrast, PepG induced IL-6 and IL-10 mRNA accumulation in both T cells and monocytes. LTA, as well as lipopolysaccharide, induced IL-6 and IL-10 mRNA production in monocytes and possibly in T cells. Whether granulocytes and B cells produce cytokines in response to bacterial stimuli remains obscure. Blockade of the CD14 receptors with monoclonal antibodies (18D11) had no influence on the PepG-induced release of TNF-α but attenuated the LTA-induced release of the same cytokine. In conclusion, our data indicate that circulating T cells and monocytes contribute to cytokine production in sepsis caused by gram-positive bacteria.
Upon invasion of mammalian tissue, bacteria activate complement and tissue macrophages. Activated macrophages secrete proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-8, which serve to recruit phagocytes from the circulation, as well as to direct an appropriate T-cell-mediated immune response (23). In blood, bacteria or bacterial products elicit a systemic inflammation characterized by massive activation of both macrophages in the reticuloendothelial system and circulating leukocytes, release of cytokines, adhesion molecule expression on endothelial cells, and development of hypotension. If not rapidly controlled, systemic inflammation may progress to sepsis, septic shock, and multiple-organ failure. In this respect, IL-10 has been shown to be an important repressor of cytokine release in patients with meningococcal sepsis (4).
Septic shock is still the major cause of death in surgical intensive care units (25). Sepsis caused by gram-negative (G−) bacteria is triggered by lipopolysaccharide (LPS). LPS in complex with LPS-binding protein binds to CD14 (33) on the surface of monocytes-macrophages and activates toll-like receptor 2 (TLR2) (17, 35), which results in the systemic release of proinflammatory mediators. The proportion of patients with sepsis caused by G+ bacteria has increased, and today G+ bacteria account for almost half of the incidents of septicemia (3, 7, 24). LPS is not found in G+ bacteria, and the chain of events that leads to G+ bacterial sepsis is largely unknown. However, cytokines are undoubtedly involved. G+ cell wall fragments, as well as the pure cell wall constituents peptidoglycan (PepG) and lipoteichoic acid (LTA), induce release of TNF-α, IL-1β, and IL-6 from cultured macrophages-monocytes (2, 14, 16, 22, 30). This induction is, however, dependent on the serum factors complement and immunoglobulins (21). Whether leukocytes of nonmyeloid origins produce cytokines in response to G+ bacterial products has not been examined.
Recently, it has been observed that PepG binds to CD14 (9). This suggests that CD14 also is involved in signaling events induced by G+ bacteria. Moreover, recent studies have shown that blockade of the CD14 receptor on murine monocytes by monoclonal antibodies (MAbs) partly inhibits PepG (12, 32)- and LTA (6, 13)-induced signaling events. This suggests that both CD14-dependent and CD14-independent signaling pathways are operating. Recent data indicate that TLR2, but not TLR4, is a signaling receptor for PepG from Staphylococcus aureus and Streptococcus pneumoniae (28, 36). In addition, there is some evidence that PepG and LTA from S. aureus act in synergy to cause multiple-organ failure and shock in rats (8).
We have recently developed a whole-blood model to study the cytokine network under both physiological and pathophysiological conditions (31). In the present study, we have examined the ability of PepG and LTA isolated from S. aureus to induce release of TNF-α, IL-6, and IL-10 in whole human blood and identified the cellular origins of these cytokines. Finally, the role of the CD14 receptor in signaling events induced by PepG or LTA was studied.
MATERIALS AND METHODS
Reagents.
Polymyxin B sulfate was purchased from Sigma-Aldrich. A MAb against CD14 (18D11) and an isotype control antibody (immunoglobulin G2a) were kind gifts from Diatec AS (Oslo, Norway). LTA from S. aureus was purchased from Sigma-Aldrich (L2515). It was prepared using a phenol extraction protocol (10). According to the manufacturer, the protein content was less than 0.5%.
Purification of PepG.
PepG was isolated from S. aureus as previously described (11). Covalently attached proteins were removed by treatment with pronase at 2 mg/ml for 1 h at 60°C (1). Anionic polymers were removed from the PepG by the treatment of purified cell walls (10 mg [dry weight]/ml) with hydrofluoric acid (48%, vol/vol) for 24 h at 4°C. The insoluble PepG was then washed by centrifugation (14,000 × g, 5 min) and resuspension once in 100 ml of Tris-HCl (pH 8.0) and five times in distilled water until the pH was neutral. The PepG was then recovered by centrifugation as described above and resuspended in saline (0.9%, wt/vol) prior to sterilization by autoclaving and storage at −20°C. PepG extract was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis with no evidence of any protein whatsoever. PepG was also enzymatically digested, and it gave the expected reversed-phase high-pressure liquid chromatography muropeptide profile with no spurious products.
Whole-blood experiments.
We have recently developed and characterized the whole-blood model used in this study (31). Briefly, venous blood from healthy volunteers was anticoagulated with Na-citrate. In experiments aimed at studying the kinetics of cytokine release, blood was incubated in Monovette syringes (Sarstedt) in the absence or presence of 10 μg of PepG or 100 μg of LTA per ml of blood, and samples were removed for analyses after 1, 3, 6, 12, and 24 h. In some experiments, blood was pretreated for 30 min with CD14 MAb 18D11 (5 μg/ml), an immunoglobulin G1 control antibody (5 μg/ml), or polymyxin B sulfate (5 μg/ml) and incubated further in the absence or presence of PepG (0.01, 0.1, 1, 3, 10, 30, or 100 μg/ml), LTA (0.01, 0.1, 1, 3, 10, 30, or 100 μg/ml), or LPS (10 ng/ml) for different periods of time, as indicated in the figure legends.
Cytokine analyses.
At indicated times, plasma was removed by centrifugation at 7,000 × g for 2 min and stored at −20°C for later analyses by an enzyme immunoassay (EIA) specific for TNF-α, IL-6, and IL-10 in accordance with protocols provided by the manufacturer (CLB, Amsterdam, The Netherlands). The detection limit was 1 pg/ml.
Fractionation of white blood cells.
CD14-positive cells were isolated from whole blood as described by Solberg et al. (29). Briefly, Dynabeads M-450 CD14 (Dynal, Oslo, Norway) were used to isolate pure CD14+ cells (monocytes, macrophages, and a subset of granulocytes) after blood incubation in the ex vivo whole-blood model. Fifty microliters of Dynabeads (4 × 108 beads/ml) were used per 500 μl of blood. The beads and blood were incubated with gentle rotation for 10 min at 4°C and subsequently placed on a magnet (MPC 6; Dynal) for 3 min. After being washed twice in cold phosphate-buffered saline, the cells were lysed by addition of 500 μl of lysis-and-binding buffer. The lysates-beads were used directly for mRNA isolation or frozen at −20°C.
CD2+ (T cells), CD15+ (granulocytes), and CD19+ (B cells) cells were isolated in a similar manner from 400, 100, and 800 μl of blood, respectively, in accordance with protocols provided by the manufacturer (Dynal).
Cytokine mRNA analyses.
Isolation of mRNA was carried out as recently described (29), using oligo(dT)25-coated Dynabeads (Dynal). Briefly, 50 μl of prewashed oligo(dT)25 (5 mg/ml)-coated Dynabeads and lysates from 400 μl of CD14+ cells, 400 μl of CD2+ cells, 100 μl of CD15+ cells, or 800 μl of CD19+ cells were rotated for 5 min at room temperature. After thorough washing, the beads-mRNA were resuspended in 20 μl of diethylpyrocarbonate-treated distilled H2O and used directly for reverse transcription (RT)-PCR or frozen at −20°C.
Semiquantitative analyses of cytokine mRNA expression were performed by RT-PCR in accordance with a previously described protocol (29). Briefly, RT-PCR was performed in a PCR cycler (GeneAmp 9600; Perkin-Elmer Cetus Corp., Norwalk, Conn.). Synthesis of cDNA was performed by RT directly on the mRNA attached to the oligo(dT)25 beads using a GeneAmp RNA PCR Kit (Perkin-Elmer Cetus Corp.). Subsequently, the cDNA pool was analyzed by PCR for cDNA specific for TNF-α, IL-6, IL-10, and β-actin using specific primers as previously described (29).
Endotoxin measurements.
Endotoxin (LPS) was measured with the Limulus amoebocyte lysate (LAL) test in accordance with the manufacturer's procedure (COATEST Endotoxin; Chromogenix, Mölndal, Sweden). Endotoxin values below 10 ng/liter were considered negative.
Statistical evaluation.
Data are presented as means ± the standard error of the mean (SEM) Student's t test or analysis of variance with Tukey post hoc assessment was used to evaluate the statistical significance of the results. Differences with P values of <0.05 were considered significant.
RESULTS
Kinetics of cytokine release in whole blood induced by PepG or LTA.
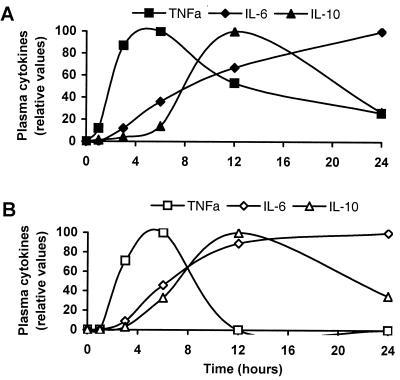
Figure 1 shows the time-dependent changes in the plasma levels of TNF-α, IL-6, and IL-10 after stimulation of whole blood with PepG (Fig. 1A) or LTA (Fig. 1B). Ten micrograms of PepG per milliliter was used because this dose gave almost maximal TNF-α values (Fig. 2). The lower potency of LTA than PepG as an inducer of TNF-α (Fig. 2) prompted us to use LTA at 100 μg/ml in the kinetics experiments. Values are normalized to peak values for each cytokine and are representative of 10 independent experiments. Spontaneous production of TNF-α, IL-6, or IL-10 was not detected in plasma from nonstimulated blood (data not shown). Addition of PepG caused a rapid increase in TNF-α to maximal values after 6 h, followed by a slow decrease. The level of IL-10 in plasma was unchanged during the first few hours of the experiment but rose at 4 to 6 h after stimulation and reached a peak after 12 h. Thereafter, a decrease was seen. In contrast, the IL-6 level increased throughout the experiment (24 h). Figure 1B shows that addition of LTA resulted in cytokine kinetics similar to those elicited by PepG; i.e., TNF-α and IL-10 were transiently released with peak values after 6 and 12 h, respectively, and IL-6 increased throughout the experiment.
FIG. 1.
Time-dependent levels of TNF-α, IL-6, and IL-10 in plasma during stimulation of whole blood with PepG (A) or LTA (B). The whole blood was added 10 μg of PepG or 100 μg of LTA per ml of blood, and the blood was incubated at 37°C for 24 h. After the indicated periods of time, plasma was isolated and analyzed for TNF-α, IL-6, and IL-10 by EIA. Cytokine values are normalized to the highest value for each cytokine in 1 representative experiment of 10 performed.
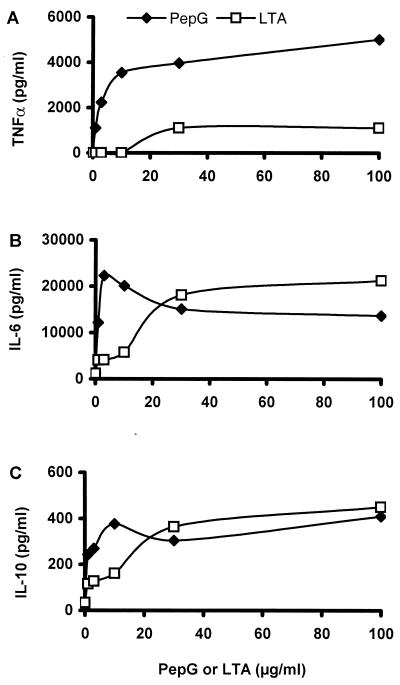
FIG. 2.
Abilities of various concentrations of PepG (closed symbols) and LTA (open symbols) to cause secretion of TNF-α (A), IL-6 (B), and IL-10 (C) in whole blood. Whole blood was spiked with various doses (0, 1, 3, 10, 30, and 100 μg/ml) of PepG or LTA and incubated for 6 h at 37°C. Plasma was analyzed for TNF-α, IL-6, and IL-10 by EIA. Results are from 1 representative experiment of 10 performed.
Dose dependency of PepG and LTA on the release of TNF-α, IL-6, and IL-10 in whole human blood.
Figure 2 shows that PepG (closed symbols) and LTA (open symbols) induced the release of TNF-α (Fig. 2A), IL-6 (Fig. 2B), and IL-10 (Fig. 2C) in a dose-dependent manner. The exact cytokine values varied between blood donors, and the results in Fig. 2 are representative of 10 experiments. As seen in Fig. 2A, PepG induced severalfold higher values of TNF-α than did LTA at all of the doses tested. The threshold doses of PepG and LTA required to induce TNF-α release were 0.1 to 1 μg of PepG per ml of blood and 10 to 30 μg of LTA per ml of blood (data not shown). Addition of 3 to 10 μg of PepG per ml gave nanogram amounts of TNF-α per milliliter of plasma, whereas an apparently high dose of LTA (30 to 100 μg/ml) was a poor inducer of TNF-α. Both PepG and LTA potently induced IL-6 and IL-10 formation. The threshold doses of the bacterial products required for the induction of IL-6 or IL-10 were in the range of 0.1 to 1 μg of PepG or LTA per ml of blood (data not shown). Figure 2B shows that concentrations of PepG of 1 to 10 μg/ml induced IL-6 values severalfold higher than those obtained by using identical concentrations of LTA, whereas PepG and LTA were fairly similar in potency at doses of 30 to 100 μg/ml. The dose-dependent effects of PepG and LTA on IL-10 production were similar to that of IL-6 (Fig. 1C). Low concentrations of PepG induced higher IL-10 values than low concentrations of LTA, but high concentrations (30 to 100 μg/ml) of LTA or PepG induced IL-10 values similar in magnitude.
Cellular origin of cytokines.
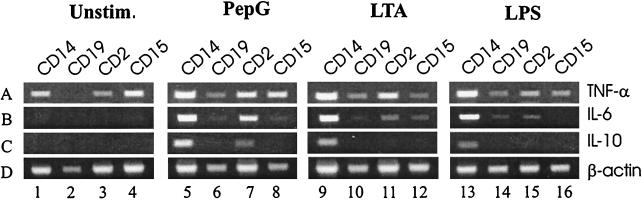
Figure 3 shows accumulation of mRNAs for TNF-α (panel A), IL-6 (panel B), IL-10 (panel C), and β-actin (panel D) in CD14+, CD19+, CD2+, and CD15+ leukocytes isolated after stimulation of whole human blood with PepG at 10 μg/ml, LTA at 30 μg/ml, or LPS at 10 ng/ml, as detected by RT-PCR. Panel A shows that TNF-α mRNA was detected in all leukocyte types from nonstimulated blood (lanes 1 to 4; by mistake, no RT product was added to lane 2), as well as from blood exposed for 6 h to PepG (lanes 5 to 8), LTA (lanes 9 to 12), or LPS (lanes 13 to 16). In contrast, IL-6 (panel B) and IL-10 (panel C) mRNAs were not detected in leukocytes isolated from nonstimulated blood (lanes 1 to 4). Panel B shows that PepG stimulation gave IL-6 mRNA accumulation in CD14+ (lane 5) and CD2+ (lane 7) cells but not in CD19+ (lane 6) and CD15+ (lane 8) cells. Addition of LTA resulted in strong induction of IL-6 mRNA in CD14+ (lane 9) cells, as well as weak signals in CD2+ (lane 11) and CD15+ (lane 12) cells. Addition of LPS resulted in high accumulation of IL-6 mRNA in CD14+ (lane 13) cells and weak signals in CD19+ (lane 14) and CD2+ (lane 15) cells. Panel C shows that addition of PepG resulted in induction of IL-10 mRNA in CD14+ (lane 5) and CD2+ (lane 7), but not in CD19+ (lane 6) and CD15+ (lane 8), cells. IL-10 mRNA accumulation induced by LTA or LPS seemed to be restricted to CD14+ cells (lanes 9 and 13, respectively).
FIG. 3.
Expression of mRNA for TNF-α, IL-6, IL-10, and β-actin in various leukocyte populations 6 h after incubation of whole blood in the absence (lanes 1 to 4) or presence (lanes 5 to 8) of PepG, LTA (lanes 9 to 12), or LPS (lanes 13 to 16) at 37°C. CD14+, CD19+, CD2+, and CD15+ cells were isolated by immunomagnetic separation. mRNAs from these cells were isolated by oligo(dT)25-coated magnetic beads, reverse transcribed, and analyzed for transcripts encoding TNF-α (panel A, 443 bp), IL-6 (panel B, 628 bp), IL-10 (panel C, 328 bp) and β-actin (panel D, 660 bp) by PCR. Note that by mistake, no RT product was added to the sample in lane 2 (panel B). Unstim., unstimulated.
Blockade of the CD14 receptor.
The results in Table 1 demonstrate the impact of blockade of the CD14 receptor with a MAb (18D11) on TNF-α formation induced by PepG or LTA. Treatment of blood for 30 min with the 18D11 CD14 antibody at 5 μg/ml prior to PepG stimulation (10 μg/ml) did not influence TNF-α values in plasma. In contrast, pretreatment with 18D11 significantly attenuated LTA (100 μg/ml)-induced TNF-α release by approximately 50% (P ≤ 0.01). In comparison, the increase in TNF-α release caused by LPS was attenuated by 70% after treatment with this CD14 MAb (P ≤ 0.001).
TABLE 1.
Effect of pretreatment of whole blood with anti-CD14 MAb 18D11 on the ability of PepG, LTA, or LPS to induce TNF-α release
| Bacterial product (concn) | Concn of TNF-α released (pg/ml)a
|
||
|---|---|---|---|
| Without 18D11 | With 18D11 | With control antibody | |
| PepG (10 μg/ml) | 6,593 ± 1,350 | 9,226 ± 1,291 | 9,558 ± 1,240 |
| LTA (30 μg/ml) | 2,506 ± 220 | 1,188 ± 269b | 2,421 ± 314 |
| LPS (10 ng/ml) | 11,140 ± 635 | 3,533 ± 636b | 10,900 ± 778 |
Values are means ± SEM of six experiments. MAb 18D11 and control antibody were added at 5 μg/ml.
Significantly lower (P < 0.05) than the value for the untreated control group as calculated by analysis of variance with Tukey post hoc assessment.
Contamination with LPS.
Table 2 shows that treatment of PepG with polymyxin B (5 μg/ml) did not influence the ability of PepG to induce TNF-α. In contrast, polymyxin B caused a 50% decrease in the TNF-α release induced by LTA (P = 0.0004) and nearly abolished the LPS-induced TNF-α release (P ≤ 0.0001). LPS activity in the PepG solution used was further found to be under the level of detection (10 ng/liter), as measured by the LAL test (data not shown). In contrast, the LTA solution demonstrated LPS activity corresponding to 4 ng of LPS per μg of LTA.
TABLE 2.
Effect of treatment of PepG, LTA, or LPS with polymyxin B on TNF-α release in whole human blood
| Bacterial product (concn) | Concn of TNF-α released (pg/ml)a
|
|
|---|---|---|
| Without polymyxin B | With polymyxin B | |
| PepG (10 μg/ml) | 6,593 ± 1,350 | 6,368 ± 1,517 |
| LTA (30 μg/ml) | 2,506 ± 220 | 1,061 ± 167b |
| LPS (10 ng/ml) | 11,140 ± 635 | 209 ± 38b |
Values are means ± SEM of six experiments.
Significantly lower (P < 0.05) than the value for the untreated control group as calculated by Student's t test.
DISCUSSION
In this paper, we demonstrate that whole human blood is a potent source of TNF-α, IL-6, and IL-10 production upon stimulation by PepG and LTA. We further present evidence that, in addition to monocytes, T cells innately produce cytokines in response to stimulation with G+ bacterial cell wall components.
The observed kinetics of TNF-α, IL-6, or IL-10 release were similar after addition of PepG or LTA and also strongly resemble the kinetics previously observed upon stimulation with LPS (P. F. Jørgensen, J. E. Wang, M. Almløf, R. Solberg, C. Okkenhaug, T. Scholz, C. Thiemermann, S. J. Foster, and A. O. Aasen, submitted for publication; 31). The amounts of PepG required to induce cytokine production were higher than those reported for LPS. Ten micrograms of PepG per milliliter of blood gave TNF-α values similar to those obtained with 1,000-fold smaller amounts of LPS. However, to compare doses of PepG and LPS is dubious because PepG is a continuous insoluble macromolecule, the ability of which to stimulate cells is known to be highly dependent on dispersion into smaller particles (27). Most notably, recent observations indicate that there exists a narrow window with respect to the molecular size of PepG molecules within which PepG can stimulate cells (P. Morreillon and P. Majcherczyk, 5th World Congr. Trauma, Shock, Inflammation and Sepsis, abstr. 354, p. 90, 2000). According to previous reports, LTA from S. aureus does not induce cytokine release from cultured monocytes (2, 16). In our model, high concentrations of LTA caused low levels of TNF-α in plasma. Thus, LTA is probably not a significant trigger for TNF-α release in vivo. However, LTA concentrations similar to those that failed to induce IL-6 release from cultured monocytes (2) induced large amounts of IL-6 in whole blood. Whether this discrepancy is attributable to IL-6 secretion from nonmonocytic cells or to paracrine factors not present in monocyte cultures remains unclear. It should be mentioned, however, that both PepG and LTA induced much higher values of IL-6 than of TNF-α and IL-10. This might indicate a role for leukocyte-derived IL-6 in bacteremia and sepsis, e.g., induction of the acute-phase response in the liver.
With regard to the cellular origins of TNF-α, we were unable to obtain conclusive data. This was partly because TNF-α mRNA was detected in all of the leukocyte populations studied but more importantly because TNF-α mRNA was spontaneously produced in the absence of a bacterial product, as detected by RT-PCR. In contrast, IL-6 and IL-10 mRNAs were not detected in unstimulated cells but were clearly induced by PepG in both monocytes and T cells in several independent experiments. To the best of our knowledge, this is the first evidence that PepG induces gene activation of IL-6 and IL-10 in T cells. The specific subpopulation of T cells with which PepG interacts and the mechanisms by which PepG interacts with T cells to induce cytokine production are, however, still unknown. The low levels of IL-6 mRNA observed in this study in T cells after stimulation with LTA and LPS were not always reproduced, and it is still unclear whether LTA and LPS also induce cytokine production in T cells. With respect to the weak and sporadic detection of IL-6 mRNA in granulocytes and B cells, further investigation is required to validate the significance of these results. A subset of neutrophils express the CD14 receptor (34), and neutrophils have been reported to produce IL-1β and TNF-α; however, conflicting data exist concerning neutrophils as IL-6 producers (reviewed in reference 5). Whether B cells contribute to cytokine production in bacteremic blood remains obscure.
Some 2 decades ago, it was reported that PepG is a human T-cell mitogen, as well as an activator of B cells (18, 26). S. aureus contains superantigens that have the potential to activate T cells in a promiscuous manner by binding to the Vβ chain of the T-cell receptor. However, we find it extremely unlikely that the effects of PepG on T cells observed in the present study were due to superantigens. Firstly, our PepG was treated with pronase. Secondly, amino acid analysis of samples has only revealed the presence of PepG amino acids. Another possible mechanism for innate stimulation of T cells by bacterial products has been suggested by Mattern and coworkers, who reported that LPS induced various activities in T cells (19) and that this stimulation depends on cell-cell interactions with monocytes via B7 molecules (20). In preliminary experiments, however, we were unable to measure by flow cytometry the expression of B7 molecules on monocytes after stimulation with LPS or PepG (P. F. Jørgensen and J. E. Wang, unpublished data).
With respect to monocytes, this is the first evidence that S. aureus PepG and LTA induce production of IL-6 and IL-10 mRNAs in circulating human cells stimulated ex vivo. It should be stressed, however, that the accumulation of IL-6 and IL-10 mRNAs in monocytes was higher and more persistent than in T cells. This is in agreement with the current opinion that monocytes and macrophages are the main innate cytokine producers in response to bacterial cell wall components in blood, as well as in tissue.
The possibility that innate T-cell responses to LPS and PepG are monocyte dependent (19), together with the finding that the innate response to PepG is serum dependent (21), highlights the importance of studying leukocyte responses in whole blood. Interleukins were originally defined and named by their function as carriers of information between leukocytes. Hence, the cytokine network is best studied in a mixed population of leukocytes. However, there is also a need to precisely define the individual actions of the various leukocyte subtypes. The model used in the present investigations meets these criteria by studying gene activation in specific leukocyte types after stimulation in whole blood.
Blockade of the CD14 receptor by MAb 18D11 did not influence the ability of PepG to induce TNF-α release. This is in contrast to previous observations that CD14 is involved in PepG signaling (12, 32). We recently demonstrated that MAb 18D11 abolished the ability of LPS to induce cytokine production in whole blood (31). However, it is not known whether LPS and PepG share binding sites on CD14 and 18D11 may block binding of LPS, but not of PepG, to the CD14 molecule. In two recent studies, it was found that transfection of TLR2, but not that of TLR1 or TLR4, conferred on various cell lines the ability to respond to S. aureus cell walls (36), as well as to isolated PepG and LTA (28). In our experiments, LTA-induced TNF-α was strongly reduced after pretreatment with 18D11, in accordance with previous findings obtained with monocytes (6). The finding that pretreatment of LTA with the LPS inhibitor polymyxin B also greatly reduced the ability of LTA to induce TNF-α release may indicate contamination of LTA with LPS. This was furthermore supported by the fact that the LTA used in our investigation demonstrated LPS activity in the LAL test. However, Jaber et al. also observed that LTA-induced TNF-α production was suppressed by 40 to 60% after polymyxin B treatment and put forward the hypothesis that LTA binds to polymyxin B (15). The LTA may also have been contaminated with PepG. However, the similar abilities of LTA and PepG to induce the release of IL-6 and IL-10 are an argument against the significance of contamination with PepG for the induction of IL-6 and IL-10. The low levels of TNF-α induced by large amounts of the LTA preparation might have been caused by contaminants. The possibility that LPS contamination contributed to the PepG effects seen in the present study is strongly contradicted by the finding that neither polymyxin B treatment nor blocking of the CD14 receptor affected the ability of PepG to induce TNF-α. Moreover, we were unable to measure any LPS in PepG by the LAL test (detection limit, 10 ng/liter).
In conclusion, our results suggest that cytokines released from circulating T cells and monocytes contribute to the pathogenesis of sepsis caused by G+ bacteria.
ACKNOWLEDGMENTS
This work was supported by the Norwegian Research Council.
We recognize the skilled technical assistance of Solveig Pettersen.
REFERENCES
- 1.Atrih A, Zöllner P, Allmaier G, Foster S J. Structural analysis of Bacillus subtilis 168 endospore peptidoglycan and its role during differentiation. J Bacteriol. 1996;178:6173–6183. doi: 10.1128/jb.178.21.6173-6183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhakdi S, Klonisch T, Nuber P, Fischer W. Stimulation of monokine production by lipoteichoic acids. Infect Immun. 1991;59:4614–4620. doi: 10.1128/iai.59.12.4614-4620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bone R C. Gram-positive organisms and sepsis. Arch Intern Med. 1994;154:26–34. [PubMed] [Google Scholar]
- 4.Brandzaeg P, Osnes L, Øvstebø R, Joø G B, Westvik Å-B, Kierulf P. Net inflammatory capacity of human septic shock plasma evaluated by a monocyte-based target cell assay: identification of interleukin-10 as a major functional deactivator of human monocytes. J Exp Med. 1996;184:51–60. doi: 10.1084/jem.184.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassatella M A. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 6.Cleveland M G, Gorham J D, Murphy T L, Tuomanen E, Murphy K M. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect Immun. 1996;64:1906–1912. doi: 10.1128/iai.64.6.1906-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen J, Abraham E. Microbiological findings and correlations with serum tumor necrosis factor-α in patients with severe sepsis and septic shock. J Infect Dis. 1999;180:116–121. doi: 10.1086/314839. [DOI] [PubMed] [Google Scholar]
- 8.de Kimpe S J, Kengatharan M, Thiemermann C, Vane J R. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc Natl Acad Sci USA. 1995;92:10359–10363. doi: 10.1073/pnas.92.22.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dziarski R, Tapping R, Tobias P S. Binding of bacterial peptidoglycan to CD14. J Biol Chem. 1998;273:8680–8690. doi: 10.1074/jbc.273.15.8680. [DOI] [PubMed] [Google Scholar]
- 10.Fischer W, Koch H U, Haas R. Improved preparation of lipoteichoic acids. Eur J Biochem. 1983;133:523–530. doi: 10.1111/j.1432-1033.1983.tb07495.x. [DOI] [PubMed] [Google Scholar]
- 11.Foster S J. Analysis of the autolysins of Bacillus subtilis 168 during vegetative growth and differentiation by using renaturing polyacrylamide gel electrophoresis. J Bacteriol. 1992;174:464–470. doi: 10.1128/jb.174.2.464-470.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta D, Kirkland T N, Viriyakosol S, Dziarski R. CD14 is a cell-activating receptor for bacterial peptidoglycan. J Biol Chem. 1996;271:23310–23316. doi: 10.1074/jbc.271.38.23310. [DOI] [PubMed] [Google Scholar]
- 13.Hattor Y, Kasai K, Akimoto K, Thiemermann C. Induction of NO synthesis by lipoteichoic acid from Staphylococcus aureus in J774 macrophages: involvement of a CD14-dependent pathway. Biochem Biophys Res Commun. 1997;233:375–379. doi: 10.1006/bbrc.1997.6462. [DOI] [PubMed] [Google Scholar]
- 14.Heumann D, Barras C, Severin A, Glauser M P, Tomasz A. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun. 1994;62:2715–2721. doi: 10.1128/iai.62.7.2715-2721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaber B L, Barrett T W, Cendoroglo N M, Sundaram S, King A J, Pereira B J. Removal of cytokine inducing substances by polymyxin-B immobilized polystyrene-derivative fibers during in vitro homoperfusion of 10% human plasma containing Staphylococcus aureus challenge. ASAIO (Am Soc Artif Intern Organs) Trans. 1998;44:48–53. doi: 10.1097/00002480-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Keller R, Fischer W, Keist R, Bassetti S. Macrophage response to bacteria: induction of marked secretory and cellular activities by lipoteichoic acids. Infect Immun. 1992;60:3664–3672. doi: 10.1128/iai.60.9.3664-3672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirschning C J, Wesche H, Merrill A T, Rothe M. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;178:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levinson A I, Dziarski A, Zweiman B, Dziarski R. Staphylococcal peptidoglycan: T-cell-dependent mitogen and relatively T-cell-independent polyclonal B-cell activator of human lymphocytes. Infect Immun. 1983;39:290–296. doi: 10.1128/iai.39.1.290-296.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattern T, Thanhauser A, Reiling N, Toellner K-M, Duchrow M, Kurumoto S, Rietschel E T, Ernst M, Brade H, Flad H-D, Ulmer A J. Endotoxin and lipid A stimulate proliferation of human T cells in the presence of autologous monocytes. J Immunol. 1994;153:2996–3004. [PubMed] [Google Scholar]
- 20.Mattern T, Flad H-D, Brade L, Rietschel E T, Ulmer A J. Stimulation of human T lymphocytes by LPS is MHC unrestricted, but strongly dependent on B7 interactions. J Immunol. 1998;160:3412–3418. [PubMed] [Google Scholar]
- 21.Mattsson E, Rollof J, Verhoef J, van Dijk H, Fleer A. Serum-induced potentiation of tumor necrosis factor alpha production by human monocytes in response to staphylococcal peptidoglycan: involvement of different serum factors. Infect Immun. 1994;62:3837–3843. doi: 10.1128/iai.62.9.3837-3843.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattsson E, Verhage L, Rollof J, Fleer A, Verhoef J, van Dijk H. Peptidoglycan and teichoic acid from Staphylococcus epidermidis stimulate human monocytes to release tumor necrosis factor-α, interleukin-β and interleukin-6. FEMS Immunol Med Microbiol. 1993;7:281–287. doi: 10.1111/j.1574-695X.1993.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 23.Metzhitov R, Janeway C A., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 24.Nogare A R. Southwest Internal Medicine conference: septic shock. Am J Med Sci. 1991;302:50–65. [PubMed] [Google Scholar]
- 25.Parillo J E. Pathogenic mechanisms of septic shock. N Engl J Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 26.Rasanen L, Arvilommi H. Cell walls, peptidoglycans, and teichoic acids of gram-positive bacteria as polyclonal inducers and immunomodulators of proliferative and lymphokine responses of human B and T lymphocytes. Infect Immun. 1982;35:523–527. doi: 10.1128/iai.35.2.523-527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenthal R S, Dziarski R. Isolation of peptidoglycan and soluble peptidoglycan fragments. Methods Enzymol. 1994;235:253–285. doi: 10.1016/0076-6879(94)35146-5. [DOI] [PubMed] [Google Scholar]
- 28.Schwander R, Dziarski R, Wesche H, Rothe M, Kirschning C J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 29.Solberg R, Scholz T, Videm V, Okkenhaug C, Aasen A O. Heparin coating reduces cell activation and mediator release in an in vitro venovenous bypass model for liver transplantation. Tranpl Int. 1998;11:252–258. doi: 10.1007/s001470050137. [DOI] [PubMed] [Google Scholar]
- 30.Timmerman C P, Mattsson E, Martinez-Martinez L, de Graaf L, van Strijp J A G, Verbrugh H A, Verhoef J, Fleer A. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun. 1993;61:4167–4172. doi: 10.1128/iai.61.10.4167-4172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, J. E., R. Solberg, C. Okkenhaug, P. F. Jørgensen, C. D. Krohn, and A. O. Aasen. Cytokine modulation in experimental endotoxemia: characterisation of an ex vivo whole blood model. Eur. Surg. Res., in press. [DOI] [PubMed]
- 32.Weidemann B, Brade H, Rietschel E T, Dziarski R, Bazil V, Kusumoto S, Flat H-D, Ulmer A J. Soluble peptidoglycan-induced monokine production can be blocked by anti-CD14 antibodies and by lipid A partial structures. Infect Immun. 1994;62:4709–4715. doi: 10.1128/iai.62.11.4709-4715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolsaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 34.Wright S D, Ramos R A, Hermanovski-Vosatka A, Rockwell P, Detmers P A. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991;173:1281–1286. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang R-B, Mark M R, Gray A, Huang A, Xie M H, Zhang M, Goddard A, Wood W I, Gurney A L, Godowski P J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimura A, Lien E, Ingalls R R, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of gram-positive bacterial cell wall components by the innate immune system occurs via toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]