FIGURE 5.
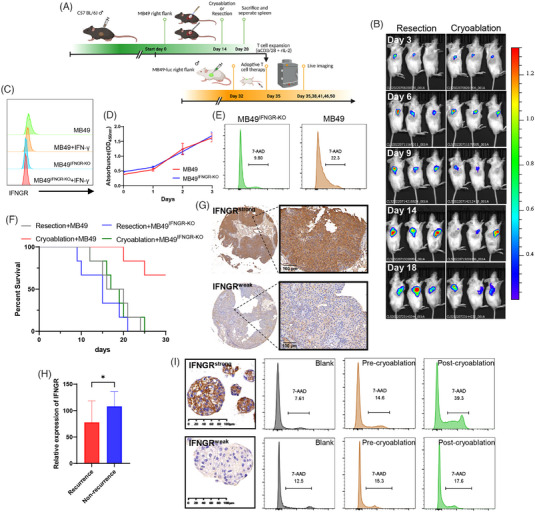
Cryoablation promoted the antitumour ability of tumour‐specific T cells and induced antitumour immunity that required IFNGR expression. (A) Schematic representation of experimental design. (B) Live imaging recording changes in subcutaneous tumours in immunodeficient mice on days 3, 6, 9, 14, and 18 after ACT via intravenous injection of activated and expanded T cells in vitro. (Left) T cells from the resection group. (Right) T cells from the cryoablation group. (C) Flow cytometric analysis of IFNGR expression in MB49, MB49IFNGR‐KO and with or without IFN‐γ stimulation (D) CCK‐8 assay showing proliferation of MB49 and MB49IFNGR‐KO (E) MB49 and MB49IFNGR‐KO cocultured with T cells collected after cryoablation. Dead cells were stained with the cell death marker 7‐Aminoactinomycin D (7‐AAD). (F) Kaplan–Meier curves of mice after MB49 or MB49IFNGR‐KO rechallenge (n = 6 per group). (G) Representative IHC staining images illustrating intratumoural IFNGR expression based on weak and strong staining intensity. (H) IFNGR expression was lower in patients who relapsed after cryoablation. Data are represented as mean ± SD, *p < .05, nonrecurrence vs. recurrence. (I) Representative IHC staining images showing that primary organoids derived from the tumour tissues of patients who relapsed after cryoablation had weak IFNGR expression compared with nonrelapse patients. Organoids with IFNGRweak and IFNGRstrong were cocultured with autologous T cells collected before and after cryoablation. Dead organoids were stained with the cell death marker 7‐Aminoactinomycin D (7‐AAD).
