Summary
Background
As the COVID-19 pandemic continues to spread, the number of associated deaths continues to increase, especially among those with pre-existing conditions. Azvudine is recommended as a priority treatment for patients with COVID-19, but its efficacy in patients with pre-existing conditions is unknown.
Methods
This is a single-centre, retrospective cohort study between December 5, 2022 and January 31, 2023 in Xiangya Hospital of Central South University in China to evaluate the clinical efficacy of Azvudine in hospitalised patients with COVID-19 and pre-existing conditions. Patients with Azvudine and controls were propensity score-matched (1:1) for age, gender, vaccination status, time from symptom onset to treatment exposure, severity at admission, concomitant treatments initiated at admission. The primary outcome was a composite outcome of disease progression, and the secondary outcome was each of these individual disease progression outcomes. The univariate Cox regression model was used to estimate a hazard ratio (HR) with 95% confidence interval (CI) for each result between the groups.
Findings
We identified 2118 hospitalised patients with COVID-19 during the study period, with a follow-up of up to 38 days. After exclusions and propensity score matching, we included 245 Azvudine recipients and 245 matched controls. Azvudine recipients had lower crude incidence rate of composite disease progression outcome compared with matched controls (7.125/1000 person-days vs. 16.004/1000 person-days, P = 0.018). There was no significant difference in all-cause death between these two groups (1.934/1000 person-days vs. 4.128/1000 person-days, P = 0.159). Azvudine treatment was associated with significantly lower risks of composite disease progression outcome compared with matched controls (HR: 0.49; 95% CI: 0.27–0.89, P = 0.016). A significant difference in all-cause death was not found (HR: 0.45; 95% CI: 0.15–1.36, P = 0.148).
Interpretation
These findings indicate that Azvudine therapy showed substantial clinical benefits in hospitalised patients with COVID-19 and pre-existing conditions, and should be considered for this population of patients.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 82103183 to F. Z., 82102803, 82272849 to G. D.), National Natural Science Foundation of Hunan Province (Grant Nos. 2022JJ40767 to F. Z., 2021JJ40976 to G. D.), Huxiang Youth Talent Program (Grant Nos. 2022RC1014 to M.S.) and Ministry of Industry and Information Technology of China (Grant Nos. TC210804V to M.S.).
Keywords: COVID-19, Pre-existing conditions, Azvudine, Real-world, Composite disease progression outcome, All-cause death
Research in context.
Evidence before this study
We searched PubMed for publications without language restriction, published before March, 28th, 2023, using search terms “SARS-CoV-2” or “COVID-19”, and “Azvudine”. A few small clinical studies compared the efficacy of Azvudine with a control cohort, yet these studies did not report the efficacy of Azvudine in hospitalized COVID-19 patients with pre-existing conditions.
Added value of this study
We conducted this propensity score-matched retrospective cohort study to investigate the efficacy of Azvudine in treating COVID-19 patients with pre-existing conditions, using the data from Xiangya Hospital. Our findings suggested that Azvudine treatment was associated with significantly lower risks of composite disease progression outcome. Sensitivity analyses were also performed and arrived at generally similar results globally.
Implications of all the available evidence
Among COVID-19 patients with pre-existing conditions, Azvudine therapy showed substantial clinical benefits in composite disease progression outcome, and should be considered for this population of patients.
Introduction
As Omicron strain of the SARS-CoV-2 virus emerge and spread, it poses a huge threat to global public health, especially in China, where COVID-19 prevention and control measures are changing.1 Patients with pre-existing comorbidities such as chronic obstructive pulmonary disease and active cancer are at increasing risk of hospitalization, re-hospitalization and COVID-19-related mortality,2,3 highlighting the significance of close attention and active treatment to those patients.
Several therapeutic agents have been authorised for the treatment of patients with COVID-19, including Paxlovid, molnupiravir and Azvudine.4, 5, 6 Paxlovid and molnupiravir have been shown to reduce hospitalization or death among patients with COVID-19 who do not require hospitalization or supplemental oxygen both in clinical trials and in real-world populations.7, 8, 9, 10, 11, 12 However, the effectiveness of Azvudine, the first Chinese oral anti-COVID-19 drug, in patients with COVID-19 was only reported in several clinical trials with small sample size. Specifically, a randomised, single-arm clinical trial on compassionate use demonstrated that Azvudine could shorten the time for nucleic acid negative conversion in 31 moderate and severe patients with COVID-19 without adverse effects.13 Another randomised, open-label, controlled clinical trial suggested that Azvudine might shorten the nucleic acid negative conversion time vs. standard antiviral treatment in the mild and common patients with COVID-19.14 Moreover, Azvudine could significantly shorten the symptom improvement time and increase the proportion of patients with COVID-19 and improved clinical symptoms in an unpublished phase 3 multicentre randomised clinical study.6 Our previous study also showed that Azvudine helped to improve the prognosis in general patients with COVID-19.15 However, whether Azvudine reduces the adverse clinical outcomes in hospitalised patients with COVID-19 with pre-existing comorbidities remains unknown.
In this retrospective cohort study, we aimed to evaluate the clinical efficacy of Azvudine in hospitalised patients with COVID-19 and pre-existing comorbidities in Xiangya Hospital, one of the largest hospitals in China. To our knowledge, this is the first real-world study to explore the use of the oral antiviral Azvudine in hospitalised patients with COVID-19 and pre-existing conditions. Two key issues were explored: Whether Azvudine is effective in treating patients with COVID-19 and pre-existing conditions in terms of composite disease progression outcome? And whether Azvudine is consistently effective in patients with different comorbidities?
Methods
Study design and patients
We performed a single-centre, retrospective cohort study in Xiangya Hospital from December 5, 2022 to January 31, 2023. We included all participants who 1) were hospitalised patients with pre-existing comorbidities and positive RT-PCR for SARS-CoV-2 infection; and 2) obtained standard treatment or Azvudine plus standard treatment. The standard treatment is based on Chinese Diagnosis and Treatment Protocol for COVID-19 (Trial Version ten) and Diagnosis and Treatment Scheme of Novel Coronavirus in Xiangya Hospital of Central South University (Trial).16,17 We excluded participants who 1) were younger than 18 years; 2) received antiviral agents other than Azvudine; or 3) who got non-invasive or invasive respiratory support on the date of admission. Our research has been approved by the institutional review committee of Xiangya Hospital, Central South University (202002024). All patients in the retrospective cohort study were anonymous and the individual informed consent was not required.
Data source
Electronic health records of patients with COVID-19 were retrieved from the inpatient system of Xiangya Hospital. The records included demographic characteristics, admission date, time from symptom onset to admission, pre-existing conditions, prescription and drug dispensing records, laboratory tests, admission to the ICU, and date of discharge or death. The health records were then linked with anonymised vaccination records provided by the Department of Immunization, Centre for Disease Control and Prevention of Hunan Province using unique identification numbers (China Identity Card). The vaccine information was accessible only in patients vaccinated in Hunan province, but not in patients vaccinated in other provinces, so the patients were marked as vaccinated status if their vaccine information was accessible, otherwise marked as unknown status. The data were consecutively collected until the planned time point.
Treatment exposure
During hospitalization, the treatment of patients with COVID-19 oral Azvudine (5 mg once a day for less than 14 days) was considered as therapeutic exposure. We defined the treatment exposure period as within the first 2 days of admission to mitigate potential immortal time bias between treatment initiation and admission.11,18 Controls were selected from the hospitalised patients with COVID-19 who did not receive Azvudine or other antiviral agents during the observation period, using propensity-score matching in a ratio of 1:1. We also tried a 1:2 matching, but the number of Azvudine recipients would drop dramatically. Since the conclusions were consistent, we just provided 1:1 matching data in the study.
Outcomes
The primary outcome was a composite outcome of disease progression including non-invasive respiratory support, initiation of endotracheal intubation, intensive care unit admission and all-cause death, and the secondary outcome was each of these individual disease progression outcomes. We collected patient outcomes from the date of admission to occurrence of outcome event, discharge date, or the date of death, whichever came first, and rates of the outcomes per 1000 person-days were analysed.
Baseline covariates
Baseline covariates of patients included age, sex, COVID-19 vaccination status (vaccinated or unknown), time from symptom onset to treatment exposure (within or beyond 5 days), severity of COVID-19 on admission (severe cases were defined as having respiratory rate ≥30 times per minute, or oxygen saturation ≤93%, or PaO2/FiO2 ≤ 300 mmHg, or lung infiltrates >50%), concomitant treatments initiated at admission (systemic steroid, antibiotics and immunomodulators). Body mass index were not included due to a large amount of missing data. Patients’ symptoms and laboratory test parameters at admission were also collected and described in our study.
Statistical analysis
The propensity-score models conditional on baseline covariates (age, sex, vaccination status, time from symptom onset to treatment, severity of COVID-19, concomitant treatments initiated at admission) was applied. We estimated the probability of receiving Azvudine through an approach of caliper matching without replacement, with a caliper width of 0.2. The balance of baseline covariates between groups before and after propensity score matching was assessed by standardised mean difference (SMD). If SMD is greater than 0.1, it means that the covariates are unbalanced.19 The univariate Cox regression model was used to estimate a HR with 95% confidence interval (CI) for each result between the groups. Subgroup analyses were performed at each level of the baseline covariates above to assess the robustness of the estimates. All statistical analyses were performed with R version 4.2.1. The level of significance was two-tailed 0.05 for statistical tests.
Role of the funding source
The funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. All the authors had full access to all the data in the study and agree to submit the manuscript for publication.
Results
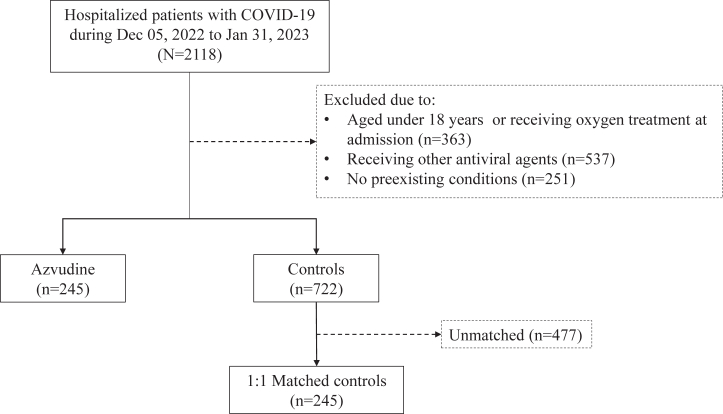
Data of 2118 hospitalised patients with confirmed diagnosis of SARS-CoV-2 infection admitted to Xiangya Hospital were consecutively collected, and with a follow-up for 38 days. Following the exclusion criteria, a total of 245 Azvudine recipients and 722 controls did not receive respiratory support therapy at baseline were eligible for inclusion (Fig. 1). Table 1 showed the baseline characteristics of Azvudine and the control groups before and after 1:1 matching of propensity score. There was no missing data in demographic characteristics, admission date, time from symptom onset to admission, pre-existing conditions, prescription and drug dispensing records, admission to the ICU, and date of discharge or death records. As for the missing data in COVID-19 vaccination status, we have adjusted the information in the propensity score matching. The covariates, coefficients, standard errors and P values of the propensity score model were shown in Supplementary Table S1. After matching, we eventually enrolled 245 Azvudine recipients and 245 matched controls, with a balanced baseline features between the two groups and the SMD lower than 0.1 (Supplementary Fig. S1). About 87.3% patients were treated with Azvudine above 5 days of the symptom's onset, and more than 64.1% patients were diagnosed as severe COVID-19 with pre-existing conditions. The laboratory parameters were shown between the groups in general. There exist missing data in laboratory tests. We have marked the number of patients in the items examined in the laboratory tests (Supplementary Table S2).
Fig. 1.
Identification of Azvudine recipients and their matched controls among hospitalised patients with COVID-19 and pre-existing conditions during the study period. Our analyses include only hospitalised patients with pre-existing comorbidities and positive RT-PCR for SARS-CoV-2 infection.
Table 1.
Baseline characteristics of the participants.
| Baseline characteristics | Before matching |
After 1:1 propensity-score matching |
||||
|---|---|---|---|---|---|---|
| Azvudine (n = 245) | Controls (n = 722) | SMD | Azvudine (n = 245) | Matched controls (n = 245) | SMD | |
| Age (years), mean (SD) | 69.13 (13.4) | 66.89 (14.8) | 0.159 | 69.13 (13.4) | 69.25 (14.0) | 0.022 |
| Gender, n (%) | 0.079 | 0.025 | ||||
| Male | 154 (62.9) | 426 (59.0) | 154 (62.9) | 157 (64.1) | ||
| Female | 91 (37.1) | 296 (41.0) | 91 (37.1) | 88 (35.9) | ||
| COVID-19 vaccination status | 0.010 | 0.016 | ||||
| Vaccinated | 118 (48.2) | 344 (47.6) | 118 (48.2) | 120 (49.0) | ||
| Unknown | 127 (51.8) | 378 (52.4) | 127 (51.8) | 125 (51.0) | ||
| Time from symptom onset to treatment exposure, n (%) | 0.334 | 0.012 | ||||
| >5 days | 214 (87.3) | 537 (74.4) | 214 (87.3) | 215 (87.8) | ||
| 0–5 days | 31 (12.7) | 185 (25.6) | 31 (12.7) | 30 (12.2) | ||
| Severity at admission, n (%) | 0.189 | 0.060 | ||||
| Non-severe | 88 (35.9) | 196 (27.1) | 88 (35.9) | 81 (33.1) | ||
| Severe | 157 (64.1) | 526 (72.9) | 157 (64.1) | 164 (66.9) | ||
| Concomitant treatments initiated at admission, n (%) | ||||||
| Systemic steroid | 64 (26.1) | 152 (21.1) | 0.119 | 64 (26.1) | 56 (22.9) | 0.076 |
| Antibiotics | 130 (53.1) | 265 (36.7) | 0.333 | 130 (53.1) | 138 (56.3) | 0.065 |
| Immunomodulators | 25 (10.2) | 57 (7.9) | 0.080 | 25 (10.2) | 22 (9.0) | 0.042 |
SMD, Standard mean difference.
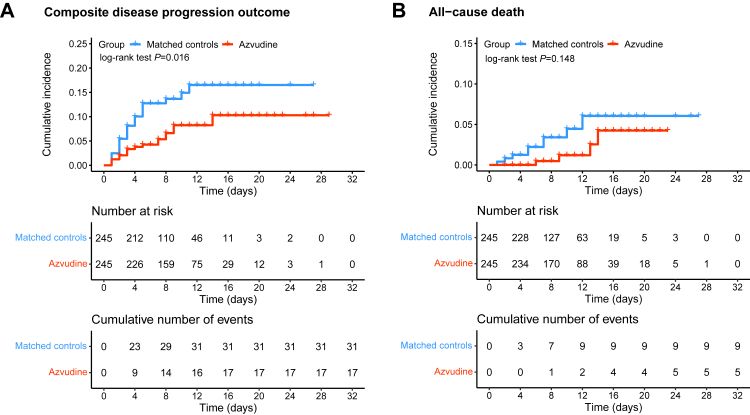
The crude incidence rate of composite disease progression outcome was 7.125 per 1000 person-days in patients treated with Azvudine vs. 16.004 per 1000 person-days in the control group (P = 0.018). The crude all-cause death rate was 1.934 per 1000 person-days among Azvudine recipients and 4.128 per 1000 person-days among the controls (P = 0.159). There were no statistical differences between these two groups in the rates of intensive care unit admission (P = 0.911), initiation of invasive mechanical ventilation (P = 0.728) and non-invasive respiratory support (P = 0.056) (Table 2). Azvudine treatment was associated with significantly lower risks of composite outcome (hazard ratio [HR]: 0.49; 95% CI: 0.27–0.89). Significant difference in all-cause death was not found (HR: 0.45; 95% CI: 0.15–1.36, P = 0.148) (Fig. 2).
Table 2.
Composite and individual outcomes in Azvudine group vs. matched controls.
| Outcome | Azvudine |
Controls |
|||
|---|---|---|---|---|---|
| n (%) | rate per 1000 person-days | n (%) | rate per 1000 person-days | Pa | |
| Composite outcome, n (%) | 17 (6.94) | 7.125 | 31 (12.65) | 16.004 | 0.018 |
| All-cause death, n (%) | 5 (2.04) | 1.934 | 9 (3.67) | 4.128 | 0.159 |
| Intensive care unit admission, n (%) | 2 (0.82) | 0.838 | 1 (0.41) | 0.516 | 0.911 |
| Initiation of invasive mechanical ventilation, n (%) | 2 (0.82) | 0.838 | 2 (0.82) | 1.033 | 0.728 |
| Non-invasive respiratory support, n (%) | 16 (6.53) | 6.706 | 26 (10.61) | 13.423 | 0.056 |
P value for log-rank test.
Fig. 2.
Cumulative incidence of composite disease progression outcome (A) and all-cause death (B) for Azvudine recipients vs. matched controls. Day 0 (baseline) represents the first day of admission to hospital. The composite disease progression outcome consists of non-invasive respiratory support, initiation of endotracheal intubation, intensive care unit admission and all-cause death.
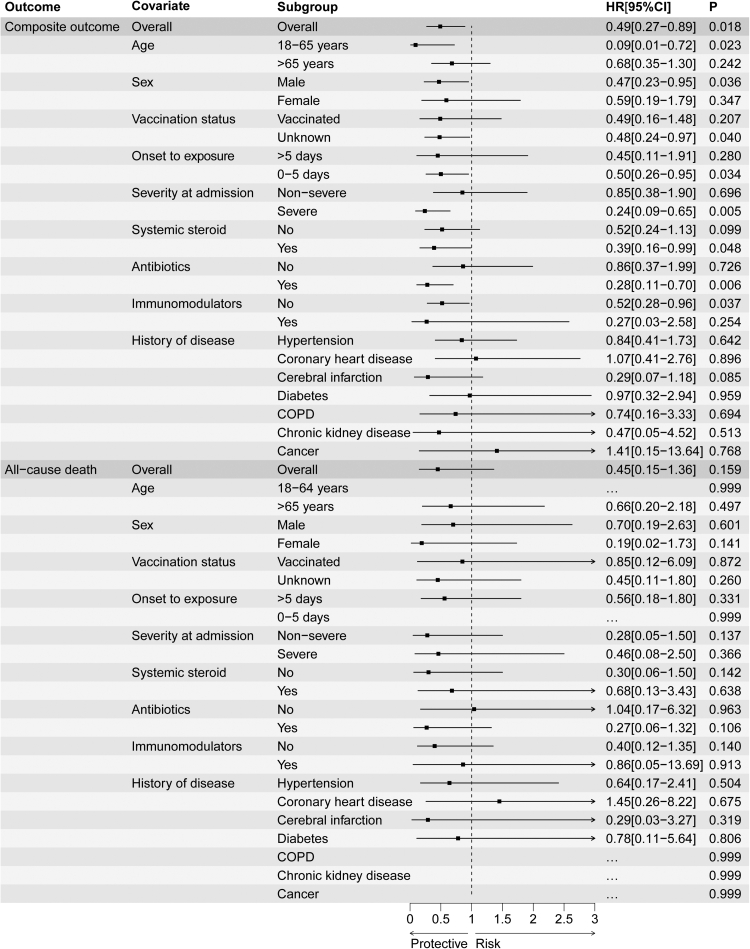
Subgroup analyses of composite disease progression outcome indicated robustness of the composite disease progression outcomes in general, and the results were significant among patients aged between 18 and 65 years, men, time from symptom onset to exposure less than 5 days, severe COVID-19 at admission, and receiving systemic steroid and antibiotics. Subgroup analyses based on comorbidities indicated robustness of the point estimates of HRs; however, the 95% CIs were broad due to the sample size. Subgroup analyses of all-cause death did not detect significant results (Fig. 3).
Fig. 3.
The effectiveness of Azvudine in reducing the risk of composite disease progression outcome and risk of all-cause death by subgroups of selected baseline characteristics. Abbreviation, COPD, chronic obstructive pulmonary disease. Ellipsis (…) means that the model does not converge due to few outcomes; Horizontal lines indicate the ranges of the 95% CIs and the vertical dash lines indicate the hazard ratio of 1.
Discussion
In this retrospective cohort study of hospitalised patients with COVID-19 and pre-existing conditions, we found that administration of Azvudine was associated with a significantly reduced risk of composite disease progression outcome, particularly in men and patients with severe COVID-19. Although the 95% CI range was wide in subgroup analyses, most of point estimates of HRs for baseline covariates and comorbidities fell to the left of 1, suggesting the protective effect of Azvudine on patients with COVID-19 and pre-existing conditions. To our best knowledge, this is the first real-world study to explore the efficacy of oral Azvudine in hospitalised patients with COVID-19 and pre-existing conditions during the pandemic wave in China.
In our study, the crude incidence rate of all-cause death was 2.04% among Azvudine recipients and 3.67% among the controls, and the rate of composite outcome of the two groups was 6.94%, and 12.65%, respectively. These results were consistent with the interim analysis of an unpublished phase 3 multi-centre randomised clinical study6 and our previous real-world study.15 Interestingly, we found that Azvudine had a stronger protective effect on severe patients with COVID-19 who were not recommended for the use of Paxlovid and molnupiravir.20,21 A previous single arm clinical trial reported that all patients with severe COVID-19 were recovered after oral Azvudine.13 Azvudine could concentrate in the thymus with its active form after oral administration, effectively inhibit the replication of SARS-CoV-2 in vivo, and protect the immune function of the thymus.13 This might explain the strong protective efficiency of Azvudine in treating severe hospitalised patients with COVID-19 and pre-existing conditions. Moreover, male recipients of Azvudine had a much better composite outcome compared with female recipients. Similar results were obtained in the previous in vitro test results of molnupiravir, another nucleoside-based RdRp inhibitor for COVID-19 treatment. Lieber et al. found that lung virus titer reductions in molnupiravir-treated males were highly significant compared to vehicle-treated males, but not in molnupiravir-treated females.22 However, the potential mechanism by which male patients with COVID-19 and benefit better from Azvudine requires further investigation.
Real-world research evidence is increasingly important for evaluating post-marketing effectiveness of drugs, which is the practice of translating the results of randomised controlled trials into real-world clinical applications.23 Patients enrolled in randomised controlled trials are highly selected with a variety of demanding conditions, for which are not comparable to more heterogeneous populations in clinical practice.24 Our real-world research included a number of patients with variable treatment patterns and various alternative interventions in practice, which could better reflect the real efficacy of Azvudine for the management of hospitalised patients with COVID-19 and pre-existing conditions. It's worth to noting that clinicians should carefully evaluate the renal function in patients with COVID-19 and chronic kidney diseases. Azvudine dose should be adjusted to 3 mg/day when eGFR is 30–60 ml/min in patients with renal insufficiency, and Azvudine is not recommended in patients with eGFR less than 30 ml/min.25
Our study also has some unavoidable limitations. Firstly, although the data from all patients with COVID-19 and were collected continuously and adjusted for possibility of selection bias and confounding factors associated with a high risk of severe COVID-19, we could not rule out the possibility of selection bias or confounding of indications in the retrospective cohort study. Secondly, we conducted a single-centre retrospective study with small sample size in Hunan province, and failed to provide data from other regions and different ethnic groups. The included cohort can only be considered to be representative in Hunan province, and studies on the efficacy of Azvudine in different regions and ethnicities should be carried out. Thirdly, Omicron was the dominant strain in China during the period, although we did not test the viral strain in these patients. Whether Azvudine is effective against other strains still needs to be investigated. Fourthly, we did not evaluate the effect of other drugs on the efficacy of Azvudine. Clinicians should pay more attention to the patient's concomitant medications and evaluate potential drug–drug interactions. Finally, we did not evaluate the adverse effects or the long-term efficacy of Azvudine. Multi-centre clinical studies with larger samples and long follow-up are needed in the future.
In conclusion, our findings suggest that Azvudine treatment in patients with COVID-19 and pre-existing conditions significantly reduces the risk of composite disease progression outcome in real-world clinical practices.
Contributors
Conception and design: Guangtong Deng, Xiang Chen, Furong Zeng, and Minxue Shen. Acquisition of data: Yuming Sun, Liping Jin, Furong Zeng, Yating Dian. Interpretation of data, statistical analysis and manuscript writing: Guangtong Deng, Furong Zeng, and Yuming Sun, Yating Dian. Revision of manuscript and administrative, technical, or material support: Guangtong Deng, Furong Zeng, Xiang Chen, Minxue Shen, Yuming Sun, Liping Jin, Yating Dian. Guangtong Deng, Furong Zeng, Xiang Chen, Minxue Shen, Yuming Sun have verified the underlying data. All the authors had full access to all the data in the study and agree to submit the manuscript for publication.
Data sharing statement
The data collected for this study, including anonymised individual patient data and a data dictionary defining each field in the data set will be made publicly available. Interested parties can contact the corresponding author (GT D).
Declaration of interests
The authors declare no conflicts of interest that pertain to this work.
Acknowledgements
We thank all the funders: the National Natural Science Foundation of China (Grant Nos. 82103183 to F. Z., 82102803, 82272849 to G. D.), Natural Science Foundation of Hunan Province (Grant Nos. 2022JJ40767 to F. Z., 2021JJ40976 to G. D.), Huxiang Youth Talent Program (Grant Nos. 2022RC1014 to M.S.) and Ministry of Industry and Information Technology of China (Grant Nos. TC210804V to M.S.). We also thank all the hospital staff members for their efforts in collecting the information that used in this study; thank the patients who participated in this study, their families, and the medical, nursing, and research staff at Xiangya Hospital of Central South University.
Footnotes
Translation: for the Chinese translation of the abstract see Supplementary Materials section.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101981.
Contributor Information
Minxue Shen, Email: shenmx1988@csu.edu.cn.
Furong Zeng, Email: zengflorachn@hotmail.com.
Xiang Chen, Email: chenxiangck@126.com.
Guangtong Deng, Email: dengguangtong@outlook.com.
Appendix A. Supplementary data
References
- 1.Joint prevention and control Mechnism of The State Council for the Novel Coronavirus Pneumonia Notice on further optimizing and implementing the prevention and control measures of the novel coronavirus. 2022. http://www.gov.cn/xinwen/2022-12/07/content_5730443.htm accessed on:
- 2.Pawlowski C., Venkatakrishnan A.J., Ramudu E., et al. Pre-existing conditions are associated with COVID-19 patients' hospitalization, despite confirmed clearance of SARS-CoV-2 virus. eClinicalMedicine. 2021;34 doi: 10.1016/j.eclinm.2021.100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Treskova-Schwarzbach M., Haas L., Reda S., et al. Pre-existing health conditions and severe COVID-19 outcomes: an umbrella review approach and meta-analysis of global evidence. BMC Med. 2021;19(1):212. doi: 10.1186/s12916-021-02058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammond J., Leister-Tebbe H., Gardner A., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu B., Chang J. The first Chinese oral anti-COVID-19 drug Azvudine launched. Innovation (Camb) 2022;3(6) doi: 10.1016/j.xinn.2022.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration Fact sheet for healthcare providers: emergency use authorization for Lagevrio (molnupiravir) capsules. 2022. https://www.fda.gov/media/155054/download accessed on:
- 8.US Food and Drug Administration Fact sheet for healthcare providers: emergency use authorization for Paxlovid. 2022. https://www.fda.gov/media/155050/download accessed on:
- 9.Infectious Diseases Society of America IDSA guidelines on the treatment and management of patients with COVID-19. 2022. https://www.idsociety.org/practice-guideline/covid-19-guidelinetreatment-and-management/ accessed on:
- 10.National Institutes of Health Therapeutic management of nonhospitalized adults with COVID-19. 2022. https://www.covid19treatmentguidelines.nih.gov/management/clinicalmanagement/nonhospitalized-adults-therapeutic-management/ accessed on:
- 11.Wong C.K.H., Au I.C.H., Lau K.T.K., Lau E.H.Y., Cowling B.J., Leung G.M. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis. 2022;22(12):1681–1693. doi: 10.1016/S1473-3099(22)00507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong C.K.H., Au I.C.H., Lau K.T.K., Lau E.H.Y., Cowling B.J., Leung G.M. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet. 2022;400(10359):1213–1222. doi: 10.1016/S0140-6736(22)01586-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J.L., Li Y.H., Wang L.L., et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct Target Ther. 2021;6(1):414. doi: 10.1038/s41392-021-00835-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren Z., Luo H., Yu Z., et al. A randomized, open-label, controlled clinical trial of Azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv Sci (Weinh) 2020;7(19) doi: 10.1002/advs.202001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen M., Xiao C., Sun Y., et al. Real-world effectiveness of Azvudine in hospitalized patients with COVID-19: a retrospective cohort study. medRxiv. 2023 doi: 10.1101/2023.01.23.23284899. [DOI] [PubMed] [Google Scholar]
- 16.General Office of the National Health Commission Diagnosis and treatment protocol for COVID-19 in China (trial version 10) 2023. http://www.gov.cn/zhengce/zhengceku/2023-01/06/content_5735343.htm accessed on:
- 17.Medical Team from Xiangya Hospital Diagnosis and treatment scheme of novel coronavirus in Xiangya Hospital of Central South University (Trial) 2023. https://mp.weixin.qq.com/s/zFcsrBK-sFwB4GNoAUDp4w accessed on:
- 18.Renoux C., Azoulay L., Suissa S. Biases in evaluating the safety and effectiveness of drugs for the treatment of COVID-19: designing real-world evidence studies. Am J Epidemiol. 2021;190(8):1452–1456. doi: 10.1093/aje/kwab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin P.C. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J. 2009;51(1):171–184. doi: 10.1002/bimj.200810488. [DOI] [PubMed] [Google Scholar]
- 20.Brown A.N., Lang Y., Zhou J., et al. Why molnupiravir fails in hospitalized patients. mBio. 2022;13(6) doi: 10.1128/mbio.02916-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J., Pan X., Zhang S., et al. Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: a multicenter randomized controlled study. Lancet Reg Health West Pac. 2023;33 doi: 10.1016/j.lanwpc.2023.100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lieber C.M., Cox R.M., Sourimant J., et al. SARS-CoV-2 VOC type and biological sex affect molnupiravir efficacy in severe COVID-19 dwarf hamster model. Nat Commun. 2022;13(1):4416. doi: 10.1038/s41467-022-32045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogelberg C., Klimek L., Bruggenjurgen B., Jutel M. Real-world evidence for the long-term effect of allergen immunotherapy: current status on database-derived European studies. Allergy. 2022;77(12):3584–3592. doi: 10.1111/all.15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makady A., de Boer A., Hillege H., Klungel O., Goettsch W. What is real-world data? A review of definitions based on literature and stakeholder interviews. Value Health. 2017;20(7):858–865. doi: 10.1016/j.jval.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y. Expert consensus on the application of Azovudine tablets in the treatment of novel coronavirus infection. Chi J China Pharm. 2023;32(3):1–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.