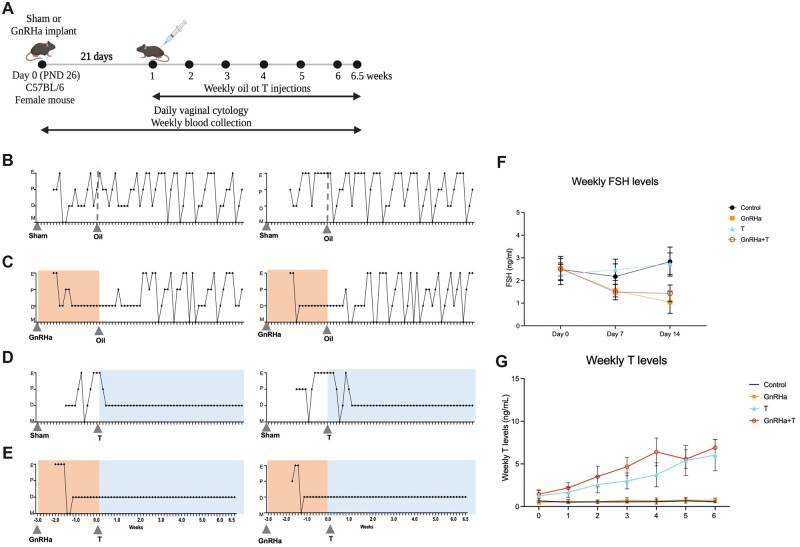
Figure 3.
Experimental design and phenotype of GnRHa+T-treated animals. (A) Experimental design for the transmasculine adolescent mouse model: GnRH analog treatment followed by T therapy, showing time of treatment and samples collection time points. (B–E) Estrus Cyclicity. (B) Control animals went through all estrous cycle phases. E, estrus; P, Proestrus; D, diestrus; M, Metestrus. (C) GnRHa-treated mice showed an expected flare and subsequent persistent diestrus, and resumed cycling after the GnRHa implant likely finished. T animals presented persistent diestrus after initiating T treatment. GnRHa+T animals showed persistent diestrus during the entire experimental period after the initial flare. (F) Weekly FSH levels were suppressed in GnRHa-treated animals. (G) Weekly testosterone levels for mice over 6 weeks of T treatment. Data are expressed as mean + SD, ANOVA followed by Tukey test.