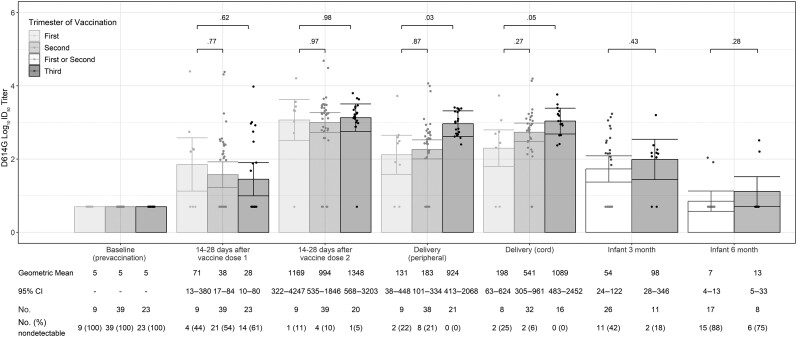
Figure 4.
Severe acute respiratory syndrome coronavirus 2 neutralizing antibody titers to D614G-like viruses among coronavirus disease 2019 (COVID-19)–vaccinated pregnant participants and their infants by trimester of receipt of first COVID-19 vaccine dose (n = 71). Fourteen COVID-19–vaccinated participants with detectable nAb to D614G-like viruses at baseline (prevaccination) were excluded from this analysis. A titer of 1:5 was assigned to participants with undetectable (<1:20) neutralizing antibody responses to vaccine. Error bars indicate the 95% confidence intervals of the log10 titers. P values compare the log10 titer between pregnant and nonpregnant participants at each timepoint. Infants of participants who received their first doses of COVID-19 vaccine during the first and second trimesters were combined because of small sample sizes (at 3 mo: 1 infant with maternal first-trimester vaccination and 25 with second-trimester vaccination; at 6 mo: 3 infants with maternal first-trimester vaccination and 15 with second-trimester vaccination). Abbreviations: CI, confidence interval; ID50, inhibitory dilution of serum samples at which relative luminescence units were reduced by 50%.