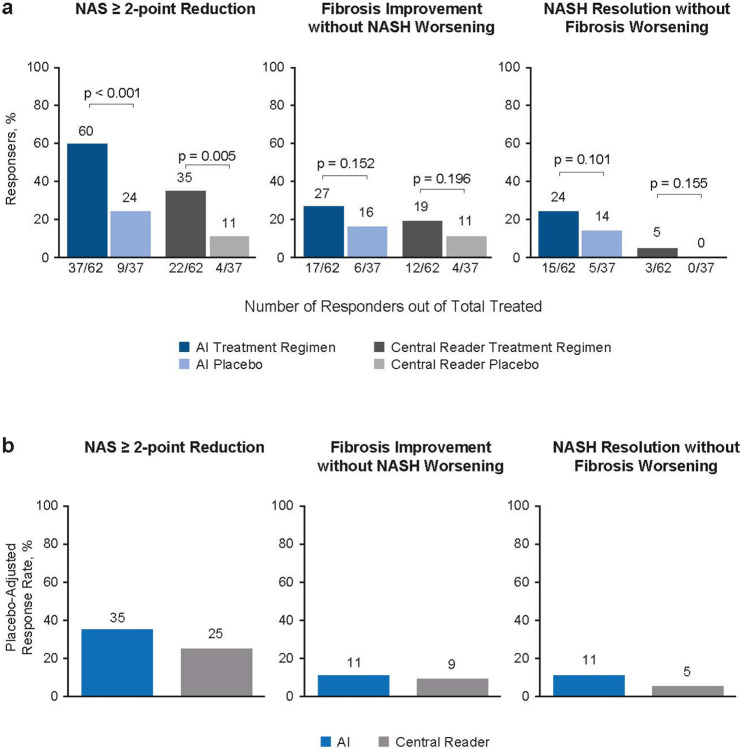
Fig. 4: AIM-based retrospective drug efficacy assessment.
AIM-NASH models were deployed on WSIs from baseline and week 48 biopsies from patients enrolled in the phase 2b ATLAS trial, which evaluated combination therapies for individuals with advanced NASH fibrosis. (a) For the trial endpoint NAS ≥ 2-point improvement, fibrosis improvement without worsening of NASH, and NASH resolution without worsening of fibrosis, AIM-NASH models showed a greater proportion of responders compared with that determined by the trial central reader. Sample sizes varied depending on data availability. (b) The placebo-adjusted response rate detected by AIM-NASH was greater than that detected by the central reader.
AI, artificial intelligence; AIM, artificial intelligence-based measurement; NAS, nonalcoholic fatty liver disease activity score; NASH, nonalcoholic steatohepatitis; WSI, whole slide image.