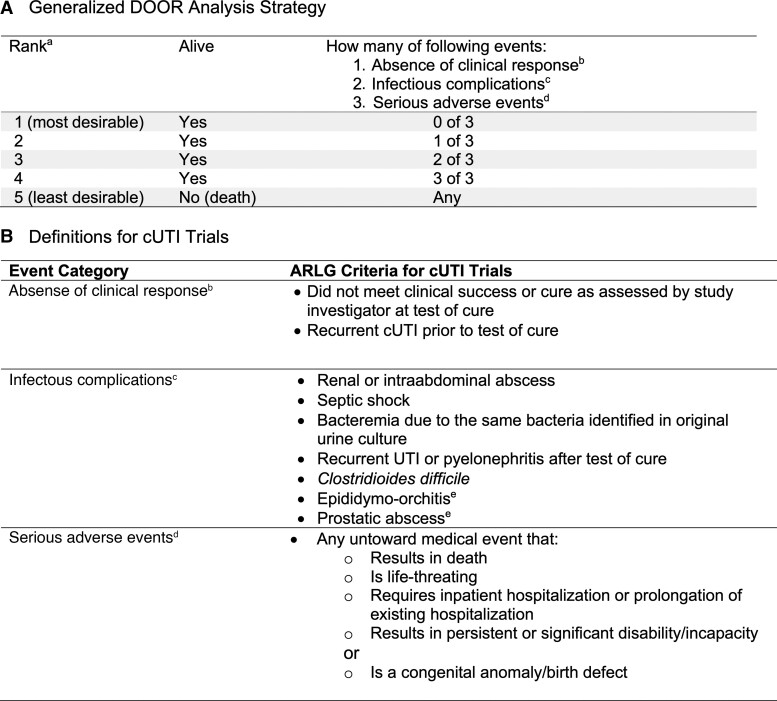
Figure 1.
DOOR analysis strategy. A, The generalized DOOR analysis strategy that could be applied to any infectious diseases clinical trial. B, Details of how the DOOR component events were defined a priori for cUTI trials. Abbreviations: ARLG, Antibacterial Resistance Leadership Group; cUTI, complicated urinary tract infection; DOOR, desirability of outcome ranking; UTI, urinary tract infection. aQuality-of-life markers, when available, could be used as a tiebreaker for patients with the same rank. bDefined as lack of global resolution of index infection or recurrence of index infection before test of cure. cDefined as a newly identified complication or progression of the original infection that was not present at enrollment, including the development of Clostridioides difficile.dDefined according to International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) E6 Good Clinical Practice guidelines. eAdded after the initial review of adverse events from the cUTI trials with agreement by the ARLG Innovations Committee.