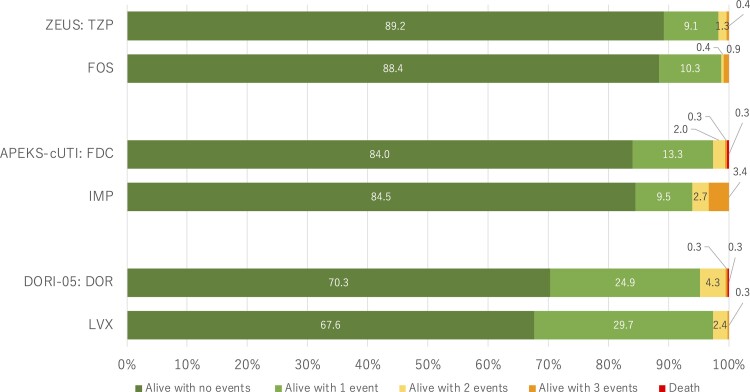
Figure 2.
Desirability of outcome ranking distribution by treatment groups for each clinical trial analyzed. The events are defined in Figure 1 and include absence of clinical response, infectious complications, serious adverse events, and death. Abbreviations: DOR, doripenem; FDC, cefiderocol; FOS, fosfomycin; IMP, imipenem-cilastatin; LVX, levofloxacin; TZP, piperacillin/tazobactam.