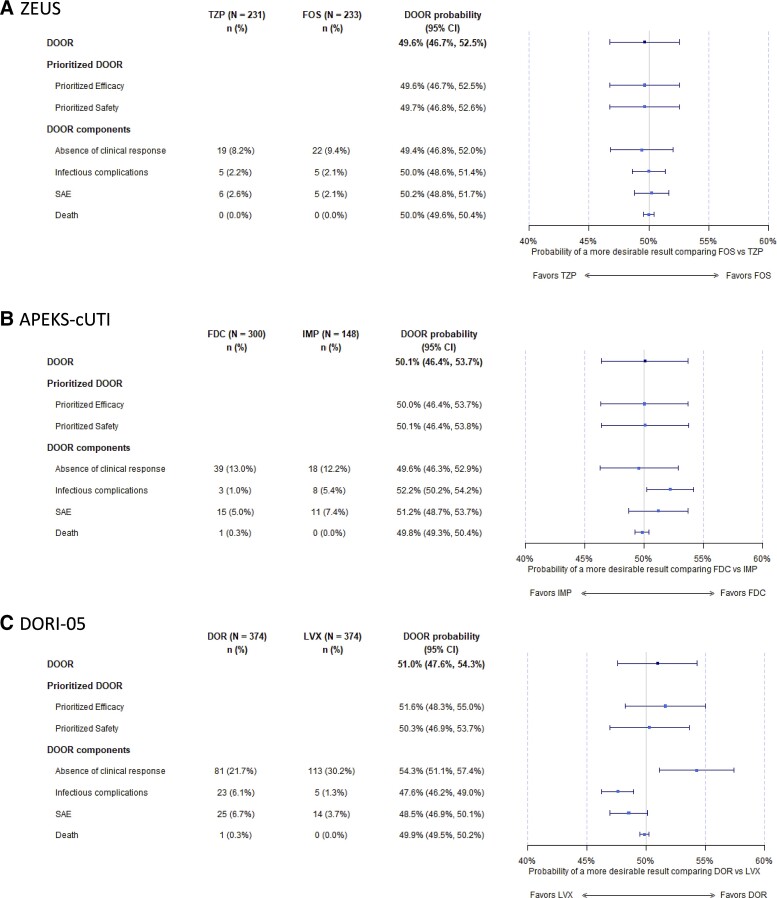
Figure 3.
Forest plot demonstrating the DOOR probabilities for the DOOR, DOOR prioritized for efficacy and safety, and the DOOR components (treatment failure, infectious complications, serious adverse events, and death) for each clinical trial (A. ZEUS; B. APEKS-cUTI; C. DORI-05). Abbreviations: CI, confidence interval; DOR, doripenem; DOOR, desirability of outcome ranking; FDC, cefiderocol; FOS, fosfomycin; IMP, imipenem-cilastatin; LVX, levofloxacin; SAE, serious adverse events; TZP, piperacillin/tazobactam.