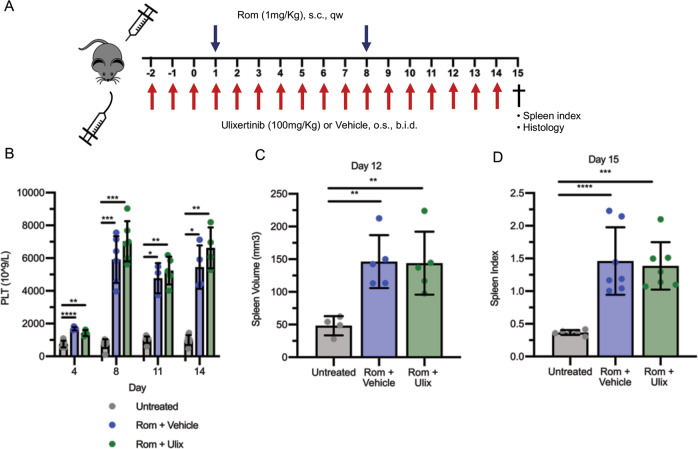
Fig. 3. ERK1/2 inhibition does not affect thrombocytosis and splenomegaly induced by Romiplostim.
A Schematic outline of the experimental design. Mice were given Romiplostim 1 mg/kg through sub-cutaneous injection once weekly. ERK1/2 inhibitor Ulixertinib 100 mg/kg or vehicle (10% DMSO in 20% SBE-β-CD in saline) was administered through oral gavage twice daily starting 3 days before the first Romiplostim injection. Mice were sacrificed after 15 days of Romiplostim treatment. B Platelet count of control mice (Untreated, in gray, n = 6–9/group), mice treated with Romiplostim alone (Rom + Vehicle, in blue, n = 4–6/group) and animals treated with Romiplostim and Ulixertinib (Rom + Ulix, in green, n = 4–6/group). Platelet count was assessed at days 4, 8, 11, and 14. Spleen volume (C) was assessed by means of Vevo2100 ultra sound system at day 12 (n = 4–5/group) while spleen index (D) was calculated at sacrifice (day 15) (n = 6–7/group). Histograms represent mean values while bars indicate the standard deviation. Comparisons were performed by means of one-way ANOVA. *: P ≤ 0.05; **: P ≤ 0.01 ***: P ≤ 0.001; ****: P ≤ 0.0001 vs Untreated. Abbreviations: s.c. sub-cutaneous; qw once weekly; o.s. oral gavage; b.i.d. twice daily; PLT platelets; Rom Romiplostim; Ulix ulixertinib.