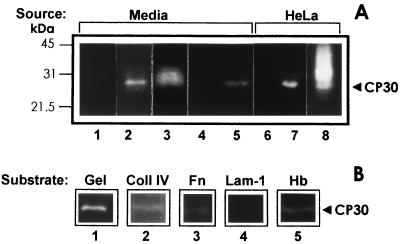
FIG. 5.
CP30 is secreted (A) and is selective for different human protein substrates (B). (A) In vitro secretion of CP30 from parasites grown in suspension was compared to trichomonads grown in coculture over a HeLa cell monolayer (2 × 106). The same amounts of parasites (10 × 106) were inoculated in 30 ml of culture media for both conditions and incubated at 37°C for 18 h to produce 40 × 106 parasites. After parasites were pelleted by centrifugation at 900 × g, 1-ml aliquots from different sample supernatants (Media) were processed for a ligand assay (see Materials and Methods). Lanes: 1, samples from control fresh media; 2, spent culture media from parasites grown in suspension; 3, spent culture media from parasites grown in coculture with fixed HeLa cell monolayers; 4 and 5, clarified supernatant (1 ml) from parasites after 30 min in suspension (control [lane 4]) or in interaction with HeLa cell monolayers (lane 5) (adherence assay) which also were processed for a ligand assay; 6 through 8, material eluted from the same amount (106) of fixed HeLa cells was loaded from a mock control without contact with parasite lysates (lane 6) or in contact with extracts from parasites grown in suspension (lane 7) or from monolayers obtained from coculture with T. vaginalis (lane 8). All samples were analyzed by substrate gelatin gel electrophoresis. The positions of the molecular size markers are on the left. (B) The CP30 proteolytic activity was tested by SDS-PAGE using different human proteins as substrates. Lanes: 1, 0.2% gelatin (Gel); 2, 800 μg of collagen IV (Coll IV); 3, 500 μg of fibronectin (Fn); 4, 500 μg of laminin-1 (Lam-1); 5, 0.2% hemoglobin (Hb). All substrate gels were activated under the same experimental conditions, 24 h at 37°C and pH 5.0.