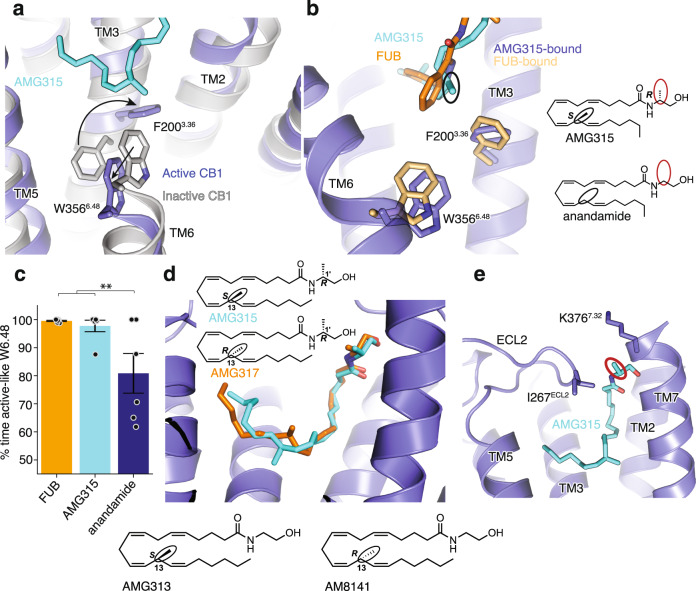
Fig. 3. CB1 “Toggle switch” interaction with ligands.
a AMG315 stabilizes the ‘toggle switch’ residues W3566.48 and F2003.36 in the active state. b Ligand interaction with residues W3566.48 and F2003.36. The methyl group in AMG315 (circled black) not present in anandamide interacts with the ‘toggle switch’. c In the simulation, anandamide stabilizes active-like conformations of W3566.48 significantly less when compared individually to FUB (MDMB-Fubinaca) (p = 0.02, two-sided Welch’s t-test) and to AMG315 (p = 0.04), as well as when compared to both FUB (MDMB-Fubinaca) and AMG315 as a group (p = 0.003). Data are presented as mean values ± SEM from n = 6 independent simulations (**p < 0.01). d Molecular docking shows that the R-stereochemistry (AMG317) instead of the S-stereochemistry at position 13 (AMG315), repositions the methyl group away from the “toggle switch”. Insert below: chemical structures of 13-methyl substituted anandamide analogs, AMG313 (13S-enantiomer) and AM8141 (13R-enantiomer). e The methyl group at position 1’ (circled red in 3B) on AMG315 interacts with residues on ECL2 (Ile267ECL2) and the extracellular region of TM7 (K3767.32). This substitution is not found in anandamide.