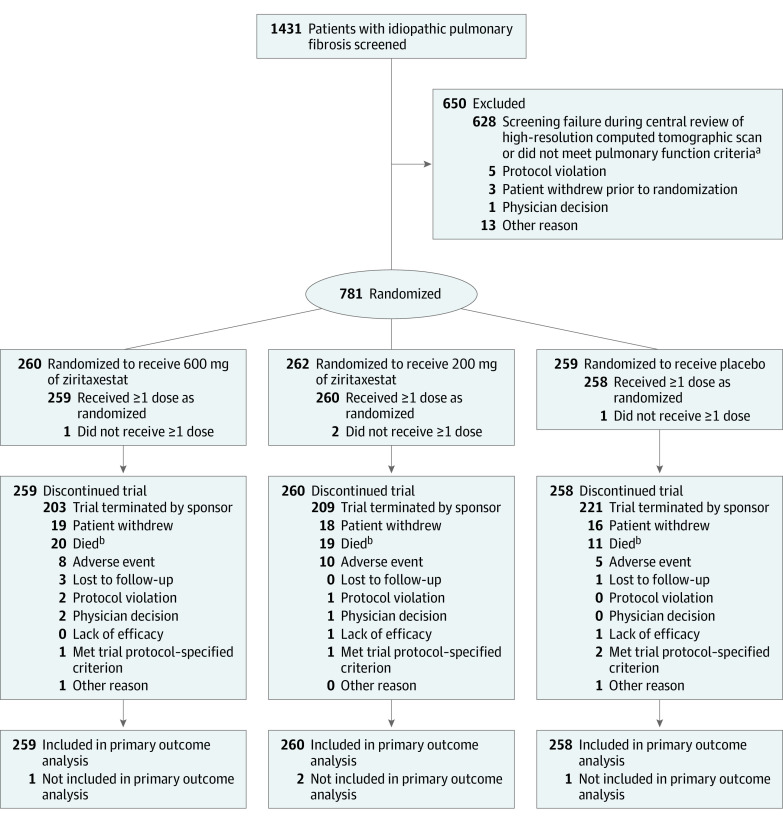
Figure 2. Screening, Exclusions, Randomization, and Flow of Patients in the ISABELA 2 Trial.
At the time of trial termination, enrollment was ongoing in the ISABELA 1 trial and had completed in the ISABELA 2 trial.
aNot meeting pulmonary function criteria was defined as a forced vital capacity (FVC) of 45% or greater than predicted of normal, a ratio of forced expiratory volume in first second of expiration to FVC of 0.7 or greater, and a diffusing capacity for carbon monoxide corrected for hemoglobin level of 30% or greater than predicted of normal.
bThe discrepancy between the number of deaths reported in this Figure and in the Results section is because of incomplete data cleaning due to the early termination of the trials. The total number who died was 24 in the 600 mg of ziritaxestat group; 22 in the 200 mg of ziritaxestat group; and 12 in the placebo group.